THE EVOLUTION OF MAN
Volume I
CHAPTER X
THE CŒLOM THEORY
The two “primary germinal layers” which
the gastræa theory has shown to be the first foundation in
the construction of the body are found in this simplest form
throughout life only in animals of the lowest grade—in the
gastræads, olynthus (the stem-form of the sponges), hydra,
and similar very simple animals. In all the other animals new
strata of cells are formed subsequently between these two primary
body-layers, and these are generally comprehended under the title
of the middle layer, or mesoderm. As a rule, the various
products of this middle layer afterwards constitute the great bulk
of the animal frame, while the original entoderm, or internal
germinal layer, is restricted to the clothing of the alimentary
canal and its glandular appendages; and, on the other hand, the
ectoderm, or external germinal layer, furnishes the outer clothing
of the body, the skin and nervous system.
In some large groups of the lower animals, such as the sponges,
corals, and flat-worms, the middle germinal layer
[ 91 ]
remains a single connected mass, and most of the body is developed from it; these have been called the three-layered metazoa, in opposition to the
two-layered animals described. Like the two-layered animals, they
have no body-cavity—that is to say, no cavity distinct from
the alimentary system. On the other hand, all the higher animals
have this real body-cavity (cœloma), and so are called
cœlomaria. In all these we can distinguish four
secondary germinal layers, which develop from the two primary
layers. To the same class belong all true vermalia (excepting the
platodes), and also the higher typical animal stems that have been
evolved from them—molluscs, echinoderms, articulates,
tunicates, and vertebrates.
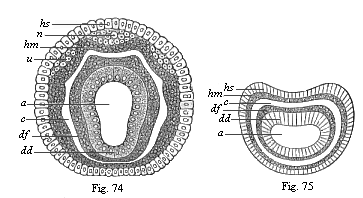
Figs. 74
and 75—Diagram of the four secondary germinal
layers, transverse section through the metazoic embryo: Fig. 74
of an annelid, Fig. 75 of a vermalian. a primitive gut,
dd ventral glandular layer, df ventral fibre-layer,
hm skin-fibre-layer, hs skin-sense-layer, u
beginning of the rudimentary kidneys, n beginning of the
nerve-plates. |
The body-cavity (cœloma) is therefore a new
acquisition of the animal body, much younger than the alimentary
system, and of great importance. I first pointed out this
fundamental significance of the cœlom in my Monograph on
the Sponges (1872), in the section which draws a distinction
between the body-cavity and the gut-cavity, and which follows
immediately on the germ-layer theory and the ancestral tree of the
animal kingdom (the first sketch of the gastræa theory). Up
to that time these two principal cavities of the animal body had
been confused, or very imperfectly distinguished; chiefly because
Leuckart, the founder of the cœlenterata group (1848), has
attributed a body-cavity, but not a gut-cavity, to these lowest
metazoa. In reality, the truth is just the other way about.
The ventral cavity, the original organ of nutrition in the
multicellular animal-body, is the oldest and most important organ
of all the metazoa, and, together with the primitive mouth, is
formed in every case in the gastrula as the primitive gut; it is
only at a much later stage that the body-cavity, which is entirely
wanting in the cœlenterata, is developed in some of the
metazoa between the ventral and the body wall. The two cavities are
entirely different in content and purport. The alimentary cavity
(enteron) serves the purpose of digestion; it contains water
and food taken from without, as well as the pulp (chymus) formed
from this by digestion. On the other hand, the body-cavity, quite
distinct from the gut and closed externally, has nothing to do with
digestion; it encloses the gut itself and its glandular appendages,
and also contains the sexual products and a certain amount of blood
or lymph, a fluid that is transuded through the ventral wall.
As soon as the body-cavity appears, the ventral wall is found to
be separated from the enclosing body-wall, but the two continue to
be directly connected at various points. We can also then always
distinguish a number of different layers of tissue in both
walls—at least two in each. These tissue-layers are formed
originally from four different simple cell-layers, which are the
much-discussed four secondary germinal layers. The outermost of
these, the skin-sense-layer (Figs. 74, 75 hs), and the
innermost, the gut-gland-layer (dd), remain at first simple
epithelia or covering-layers. The one covers the outer surface of
the body, the other the inner
[ 92 ]
surface of the ventral wall; hence they are called
confining or limiting layers. Between them are the two
middle-layers, or mesoblasts, which enclose the body-cavity.
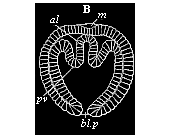 Fig.
76—Cœlomula of sagitta (gastrula with a
couple of cœlom-pouches. (From Kowalevsky.)
bl.p primitive mouth, al primitive gut, pv
cœlom-folds, m permanent mouth.
Fig.
76—Cœlomula of sagitta (gastrula with a
couple of cœlom-pouches. (From Kowalevsky.)
bl.p primitive mouth, al primitive gut, pv
cœlom-folds, m permanent mouth. |
The four secondary germinal layers are so distributed in the
structure of the body in all the cœlomaria (or all metazoa
that have a body-cavity) that the outer two, joined fast together,
constitute the body-wall, and the inner two the ventral wall; the
two walls are separated by the cavity of the cœlom. Each of
the walls is made up of a limiting layer and a middle layer. The
two limiting layers chiefly give rise to epithelia, or
covering-tissues, and glands and nerves, while the middle layers
form the great bulk of the fibrous tissue, muscles, and connective
matter. Hence the latter have also been called fibrous or muscular
layers. The outer middle layer, which lies on the inner side of the
skin-sense-layer, is the skin fibre-layer; the inner middle layer,
which attaches from without to the ventral glandular layer, is the
ventral fibre layer. The former is usually called briefly the
parietal, and the latter the visceral layer or mesoderm. Of the
many different names that have been given to the four secondary
germinal layers, the following are those most in use
to-day:—
1. Skin-sense-layer
(outer limiting layer). |
I. Neural layer
(neuroblast). |
The two secondary
germinal
layers of the body-wall:
I. Epithelial.
II. Fibrous. |
2. Skin-fibre-layer
(outer middle layer). |
II. Parietal layer
(myoblast). |
3. Gut-fibre-layer
(inner middle layer). |
III. Visceral layer
(genoblast). |
The two secondary
germinal
layers of the gut-wall:
III. Fibrous.
IV. Epithelial. |
4. Gut-gland-layer
(inner limiting layer). |
IV. Enteral layer
(enteroblast) |
The first scientist to recognise and clearly distinguish the
four secondary germinal layers was Baer. It is true that he was not
quite clear as to their origin and further significance, and made
several mistakes in detail in explaining them. But, on the whole,
their great importance did not escape him. However, in later years
his view had to be given up in consequence of more accurate
observations. Remak then propounded a three-layer theory, which was
generally accepted. These theories of cleavage, however, began to
give way thirty years ago, when Kowalevsky (1871) showed that in
the case of Sagitta (a very clear and typical subject of
gastrulation) the two middle germinal layers and the two limiting
layers arise not by cleavage, but by folding—by a secondary
invagination of the primary inner germ-layer. This invagination or
folding proceeds from the primitive mouth, at the two sides of
which (right and left) a couple of pouches are formed. As these
cœlom-pouches or cœlom-sacs detach themselves from the
primitive gut, a double body-cavity is formed (Figs. 74–76).
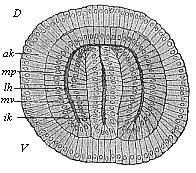 Fig.
77—Cœlomula of sagitta, in section. (From
Hertwig.) D dorsal side, V ventral side,
ik inner germinal layer, mv visceral mesoblast,
lh body-cavity, mp parietal mesoblast, ak outer
germinal layer.
Fig.
77—Cœlomula of sagitta, in section. (From
Hertwig.) D dorsal side, V ventral side,
ik inner germinal layer, mv visceral mesoblast,
lh body-cavity, mp parietal mesoblast, ak outer
germinal layer. |
The same kind of cœlom-formation as in sagitta was
afterwards found by Kowalevsky in brachiopods and other
invertebrates, and in the lowest vertebrate—the amphioxus.
Further instances were discovered by two English embryologists, to
whom we owe very considerable advance in ontogeny—E.
Ray-Lankester and F. Balfour. On the strength of these and other
studies, as well as most extensive research of their own, the
brothers Oscar and Richard Hertwig constructed in 1881
[ 93 ]
the Cœlom Theory. In order to appreciate fully
the great merit of this illuminating and helpful theory, one must
remember what a chaos of contradictory views was then represented
by the “problem of the mesoderm,” or the much-disputed
“question of the origin of the middle germinal layer.”
The cœlom theory brought some light and order into this
infinite confusion by establishing the following points: 1. The
body-cavity originates in the great majority of animals (especially
in all the vertebrates) in the same way as in sagitta: a couple of
pouches or sacs are formed by folding inwards at the primitive
mouth, between the two primary germinal layers; as these pouches
detach from the primitive gut, a pair of cœlom-sacs (right
and left) are formed; the coalescence of these produces a simple
body-cavity. 2. When these cœlom-embryos develop, not as a
pair of hollow pouches, but as solid layers of cells (in the shape
of a pair of mesodermal streaks)—as happens in the higher
vertebrates—we have a secondary (cenogenetic) modification of
the primary (palingenetic) structure; the two walls of the pouches,
inner and outer, have been pressed together by the expansion of the
large food-yelk. 3. Hence the mesoderm consists from the first of
two genetically distinct layers, which do not originate by
the cleavage of a primary simple middle layer (as Remak supposed).
4. These two middle layers have, in all vertebrates, and the great
majority of the invertebrates, the same radical significance for
the construction of the animal body; the inner middle layer, or the
visceral mesoderm, (gut-fibre layer), attaches itself to the
original entoderm, and forms the fibrous, muscular, and connective
part of the visceral wall; the outer middle layer, or the parietal
mesoderm (skin-fibre-layer), attaches itself to the original
ectoderm and forms the fibrous, muscular, and connective part of
the body-wall. 5. It is only at the point of origination, the
primitive mouth and its vicinity, that the four secondary germinal
layers are directly connected; from this point the two middle
layers advance forward separately between the two primary germinal
layers, to which they severally attach themselves. 6. The further
separation or differentiation of the four secondary germinal layers
and their division into the various tissues and organs take place
especially in the later fore-part or head of the embryo, and extend
backwards from there towards the primitive mouth.
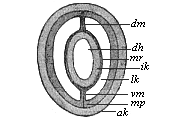 Fig.
78—Section of a young sagitta. (From
Hertwig.) dh visceral cavity, ik and ak
inner and outer limiting layers, mv and mp inner and
outer middle layers, lk body-cavity, dm and vm
dorsal and visceral mesentery.
Fig.
78—Section of a young sagitta. (From
Hertwig.) dh visceral cavity, ik and ak
inner and outer limiting layers, mv and mp inner and
outer middle layers, lk body-cavity, dm and vm
dorsal and visceral mesentery. |
All animals in which the body-cavity demonstrably arises in this
way from the primitive gut (vertebrates, tunicates, echinoderms,
articulates, and a part of the vermalia) were comprised by the
Hertwigs under the title of enterocœla, and were contrasted
with the other groups of the pseudocœla (with false
body-cavity) and the cœlenterata (with no body-cavity).
However, this radical distinction and the views as to
classification which it occasioned have been shown to be untenable.
Further, the absolute differences in tissue-formation which the
Hertwigs set up between the enterocœla and pseudocœla
cannot be sustained in this connection. For these and other reasons
their cœlom-theory has been much criticised and partly
abandoned. Nevertheless, it has rendered a great and lasting
service in the solution of the difficult problem of the mesoderm,
and a material part of it will certainly be retained. I consider it
an especial merit of the theory that it has established the
identity of the development of the two middle layers in all the
vertebrates, and has traced them as cenogenetic modifications back
to the original palingenetic form of development that we still find
in the amphioxus. Carl Rabl comes to the same conclusion in his
able Theory of the Mesoderm, and so do Ray-Lankester, Rauber,
Kupffer, Ruckert, Selenka, Hatschek, and others. There is a general
agreement in these and many other recent writers that all the
different forms of cœlom-construction, like those of
gastrulation, follow one and the same strict hereditary law in the
vast vertebrate stem; in spite of their apparent differences,
they
[ 94 ]
are all only cenogenetic modifications of one
palingenetic type, and this original type has been preserved for us
down to the present day by the invaluable amphioxus.
But before we go into the regular cœlomation of the
amphioxus, we will glance at that of the arrow-worm
(Sagitta), a remarkable deep-sea worm that is interesting in
many ways for comparative anatomy and ontogeny. On the one hand,
the transparency of the body and the embryo, and, on the other
hand, the typical simplicity of its embryonic development, make the
sagitta a most instructive object in connection with various
problems. The class of the chætogatha, which is only
represented by the cognate genera of Sagitta and
Spadella, is in another respect also a most remarkable branch
of the extensive vermalia stem. It was therefore very gratifying
that Oscar Hertwig (1880) fully explained the anatomy,
classification, and evolution of the chætognatha in his
careful monograph.
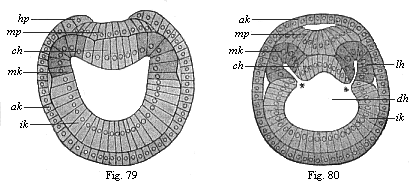
Figs. 79 and
80.—Transverse section of amphioxus-larvæ.
(From Hatschek.) Fig. 79 at the commencement of cœlom
formation (still without segments), Fig. 80 at the stage with four
primitive segments. ak, ik, mk outer, inner, and middle
germinal layer, hp horn plate, mp medullary plate,
ch chorda, * and * disposition of the cœlom-pouches,
lh body-cavity.) |
The spherical blastula that arises from the impregnated ovum of
the sagitta is converted by a folding at one pole into a typical
archigastrula, entirely similar to that of the Monoxenia
which I described (Chapter VIII, Fig. 29). This oval, uni-axial
cup-larva (circular in section) becomes bilateral (or tri-axial) by
the growth of a couple of cœlom-pouches from the primitive
gut (Figs. 76, 77). To the right and left a sac-shaped fold appears
towards the top pole (where the permanent mouth, m,
afterwards arises). The two sacs are at first separated by a couple
of folds of the entoderm (Fig. 76
pv), and are still connected with the primitive gut by wide
apertures; they also communicate for a short time with the dorsal
side (Fig. 77 d). Soon, however, the cœlom-pouches
completely separate from each other and from the primitive gut; at
the same time they enlarge so much that they close round the
primitive gut (Fig. 78). But in the middle
line of the dorsal and ventral sides the pouches remain separated,
their approaching walls joining here to form a thin vertical
partition, the mesentery (dm and vm). Thus
Sagitta has throughout life a double body-cavity (Fig. 78
lk), and the gut is fastened to the body-wall both above and
below by a mesentery—below by the ventral mesentery
(vm), and above by the dorsal mesentery (dm). The
inner layer of the two cœlom-pouches (mv) attaches
itself to the entoderm (ik), and forms with it the visceral
wall. The outer layer (mp) attaches itself to the ectoderm
(ak), and forms with it the outer body-wall. Thus we have in
Sagitta a perfectly clear and simple illustration of the
original cœlomation of the enterocœla. This
palingenetic fact is the more important, as the greater part of the
two body-cavities in Sagitta changes afterwards into sexual
glands—the fore or female part into a pair of ovaries, and
the hind or male part into a pair of testicles.
Cœlomation takes place with equal clearness and
transparency in the case of
[ 95 ]
the amphioxus, the lowest vertebrate, and its
nearest relatives, the invertebrate tunicates, the sea-squirts.
However, in these two stems, which we class together as
Chordonia, this important process is more complex, as two other
processes are associated with it—the development of the
chorda from the entoderm and the separation of the medullary plate
or nervous centre from the ectoderm. Here again the skulless
amphioxus has preserved to our own time by tenacious heredity the
chief phenomena in their original form, while it has been more or
less modified by embryonic adaptation in all the other vertebrates
(with skulls). Hence we must once more thoroughly understand the
palingenetic embryonic features of the lancelet before we go on to
consider the cenogenetic forms of the craniota.
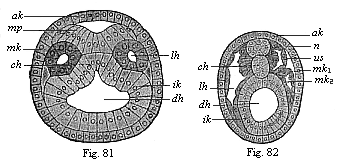
Figs. 81 and
82.—Transverse section of amphioxus embryo. Fig.
81 at the stage with five somites, Fig. 82 at the stage with eleven
somites. (From Hatschek.) ak outer germinal layer,
mp medullary plate, n nerve-tube, ik inner
germinal layer, dh visceral cavity, lh body-cavity,
mk middle germinal layer (mk1 parietal,
mk2 visceral), us primitive segment,
ch chorda. |
The cœlomation of the amphioxus, which was first observed
by Kowalevsky in 1867, has been very carefully studied since by
Hatschek (1881). According to him, there are first formed on the
bilateral gastrula we have already considered (Figs. 36, 37) three
parallel longitudinal folds—one single ectodermal fold in the
central line of the dorsal surface, and a pair of entodermic folds
at the two sides of the former. The broad ectodermal fold that
first appears in the middle line of the flattened dorsal surface,
and forms a shallow longitudinal groove, is the beginning of the
central nervous system, the medullary tube. Thus the primary outer
germinal layer divides into two parts, the middle medullary plate
(Fig. 81 mp) and the horny-plate (ak), the beginning
of the outer skin or epidermis. As the parallel borders of the
concave medullary plate fold towards each other and grow underneath
the horny-plate, a cylindrical tube is formed, the medullary tube
(Fig. 82 n); this quickly detaches itself altogether from
the horny-plate. At each side of the medullary tube, between it and
the alimentary tube (Figs. 79–82 dh), the two parallel
longitudinal folds grow out of the dorsal wall of the alimentary
tube, and these form the two cœlom-pouches (Figs. 80, 81
lh). This part of the entoderm, which thus represents the first
structure of the middle germinal layer, is shown darker than the
rest of the inner germinal layer in Figs. 79–82. The edges of
the folds meet, and thus form closed tubes (Fig. 81 in
section).
During this interesting process the outline of a third very
important organ, the chorda or axial rod, is being formed between
the two cœlom-pouches. This first foundation of the skeleton,
a solid cylindrical cartilaginous rod, is formed in the middle line
of the dorsal primitive gut-wall, from the entodermal cell-streak
that remains here between the two cœlom-pouches (Figs.
79–82 ch). The chorda appears at first in the shape of
a flat longitudinal fold or a shallow groove (Figs. 80, 81); it
does not become a solid cylindrical cord until after separation
from the primitive gut (Fig. 82). Hence we might say that the
dorsal wall of the primitive gut forms three parallel longitudinal
folds at this important period—one single fold and a pair of
folds. The single middle fold becomes the chorda, and lies
immediately below the groove of the ectoderm, which becomes the
medullary
[ 96 ]
tube; the pair of folds to the right and left lie at
the sides between the former and the latter, and form the
cœlom-pouches. The part of the primitive gut that remains
after the cutting off of these three dorsal primitive organs is the
permanent gut; its entoderm is the gut-gland-layer or enteric
layer.
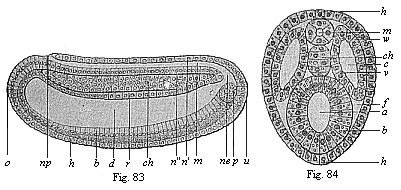
Figs. 83 and
84—Chordula of the amphioxus. Fig. 83 median
longitudinal section (seen from the left). Fig. 84 transverse
section. (From Hatschek.) In Fig. 83 the cœlom-pouches
are omitted, in order to show the chordula more clearly. Fig. 84 is
rather diagrammatic. h horny-plate, m medullary tube,
n wall of same (n' dorsal, n" ventral),
ch chorda, np neuroporus, ne canalis
neurentericus, d gut-cavity, r gut dorsal wall,
b gut ventral wall, z yelk-cells in the latter, u
primitive mouth, o mouth-pit, p promesoblasts
(primitive or polar cells of the mesoderm), w parietal
layer, v visceral layer of the mesoderm, c
cœlom, f rest of the segmentation-cavity. |
|
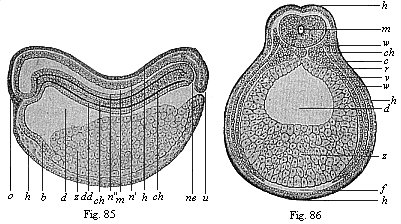
Figs. 85 and
86—Chordula of the amphibia (the ringed adder).
(From Goette.) Fig. 85 median longitudinal section (seen
from the left), Fig. 86 transverse section (slightly diagrammatic).
Lettering as in Figs. 83 and 84. |
I give the name of chordula or chorda-larva to the
embryonic stage of the vertebrate organism which is represented by
the amphioxus larva at this period (Figs. 83, 84, in the third
period of development according to Hatschek). (Strabo and Plinius
give the name of cordula or cordyla to young fish
larvæ.) I ascribe the utmost phylogenetic significance to it,
as it is found in all the chorda-animals (tunicates as well as
vertebrates) in essentially the same form. Although the
accumulation of food-yelk greatly modifies the form of the chordula
in the higher vertebrates, it remains the same in its main features
throughout. In all
[ 97 ]
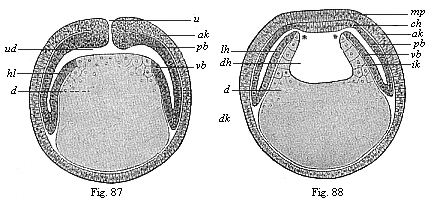
Figs. 87 and
88—Diagrammatic vertical section of
cœlomula-embryos of vertebrates. (From Hertwig.)
Fig. 87, vertical section through the primitive mouth, Fig.
88, vertical section before the primitive mouth. u
primitive mouth, ud primitive gut. d yelk, dk
yelk-nuclei, dh gut-cavity, lh body-cavity, mp
medullary plate, ch chorda plate, ak and ik
outer and inner germinal layers, pb parietal and vb
visceral mesoblast. |
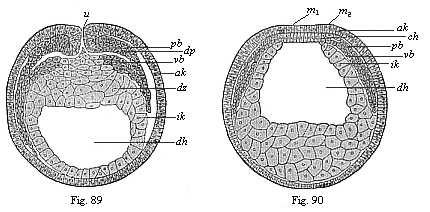
Figs. 89 and
90—Transverse section of cœlomula embryos of
triton. (From Hertwig.) Fig. 89, section through
the primitive mouth. Fig. 90, section in front of the primitive
mouth, u primitive mouth. dh gut-cavity, dz
yelk-cells, dp yelk-stopper, ak outer and ik
inner germinal layer, pb parietal and vb visceral
middle layer, m medullary plate, ch chorda. |
cases the nerve-tube (m) lies on the dorsal
side of the bilateral, worm-like body, the gut-tube (d) on
the ventral side, the chorda (ch) between the two, on the
long axis, and the cœlom pouches (c) at each side. In
every case these primitive organs develop in the same way from the
germinal layers, and the same organs always arise from them in the
mature chorda-animal. Hence we may conclude, according to the laws
of the theory of descent, that all these chordonia or chordata
(tunicates and vertebrates) descend from an ancient common
ancestral form, which we may call Chordæa. We should
regard this long-extinct Chordæa, if it were still in
existence, as a special class of unarticulated worm
(chordaria). It is especially noteworthy that neither the
dorsal nerve-tube nor the ventral gut-tube, nor even the chorda
that lies between them, shows any trace of articulation or
segmentation; even the two cœlom-sacs are not segmented at
first (though in the amphioxus they quickly divide into a series of
parts by transverse
[ 98 ]
folding). These ontogenetic facts are of the
greatest importance for the purpose of learning those ancestral
forms of the vertebrates which we have to seek in the group of the
unarticulated vermalia. The cœlom-pouches were originally
sexual glands in these ancient chordonia.
|
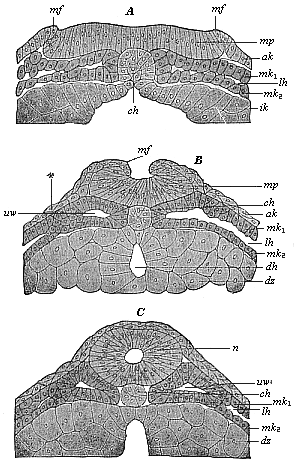
Fig. 91. A,
B, C.—Vertical section of the dorsal part of three
triton-embryos. (From Hertwig.) In Fig. A the
medullary swellings (the parallel borders of the medullary plate)
begin to rise; in Fig. B they grow towards each other; in
Fig. C they join and form the medullary tube. mp
medullary plate, mf medullary folds, n nerve-tube,
ch chorda, lh body-cavity, mk1 and
mk2 parietal and visceral mesoblasts, uv
primitive-segment cavities, ak ectoderm, ik entoderm,
dz yelk-cells, dh gut-cavity. |
From the evolutionary point of view the cœlom-pouches are,
in any case, older than the chorda; since they also develop in the
same way as in the chordonia in a number of invertebrates which
have no chorda (for instance, Sagitta, Figs. 76–78).
Moreover, in the amphioxus the first outline of the chorda appears
later than that of the cœlom-sacs. Hence we must, according
to the biogenetic law, postulate a special intermediate form
between the gastrula and the chordula, which we will call
cœlomula, an unarticulated, worm-like body with primitive
gut, primitive mouth, and a double body-cavity, but no chorda. This
embryonic form, the bilateral cœlomula (Fig. 81), may in turn be regarded as the
ontogenetic reproduction (maintained by heredity) of an ancient
ancestral form of the cœlomaria, the
Cœlomæa (cf. Chapter XX).
In Sagitta and other worm-like animals the two
cœlom-pouches (presumably gonads or sex-glands) are separated
by a complete median partition, the dorsal and ventral mesentery (Fig. 78 dm, vm); but in the
vertebrates only the upper part of this vertical partition is
maintained, and forms the dorsal mesentery. This mesentery
afterwards takes the form of a thin membrane, which fastens the
visceral tube to the chorda (or the vertebral column). At the under
side of the visceral tube the cœlom-sacs blend together,
their inner or median walls breaking down and disappearing. The
body-cavity then forms a single simple hollow, in which the gut is
quite free, or only attached to the dorsal wall by means of the
mesentery.
The development of the body-cavity and the formation of the
chordula in the higher vertebrates is, like that of the
gastrula, chiefly modified by the pressure of the food-yelk on
the embryonic structures, which forces its hinder part into
[ 99 ]
a discoid expansion. These cenogenetic modifications
seem to be so great that until twenty years ago these important
processes were totally misunderstood. It was generally believed
that the body-cavity in man and the higher vertebrates was due to
the division of a simple middle layer, and that the latter arose by
cleavage from one or both of the primary germinal layers. The truth
was brought to light at last by the comparative embryological
research of the Hertwigs. They showed in their Cœlom
Theory (1881) that all vertebrates are true enterocœla,
and that in every case a pair of cœlom-pouches are developed
from the primitive gut by folding. The cenogenetic chordula-forms
of the craniotes must therefore be derived from the palingenetic
embryology of the amphioxus in the same way as I had previously
proved for their gastrula-forms.
The chief difference between the cœlomation of the acrania
(amphioxus) and the other vertebrates (with
skulls—craniotes) is that the two cœlom-folds of the
primitive gut in the former are from the first hollow vesicles,
filled with fluid, but in the latter are empty pouches, the layers
of which (inner and outer) close with each other. In common
parlance we still call a pouch or pocket by that name, whether it
is full or empty. It is different in ontogeny; in some of our
embryological literature ordinary logic does not count for very
much. In many of the manuals and large treatises on this science it
is proved that vesicles, pouches, or sacs deserve that name only
when they are inflated and filled with a clear fluid. When they are
not so filled (for instance, when the primitive gut of the gastrula
is filled with yelk, or when the walls of the empty
cœlom-pouches are pressed together), these vesicles must not
be cavities any longer, but “solid structures.”
The accumulation of food-yelk in the ventral wall of the
primitive gut (Figs. 85, 86) is the simple
cause that converts the sac-shaped cœlom-pouches of the
acrania into the leaf-shaped cœlom-streaks of the craniotes.
To convince ourselves of this we need only compare, with Hertwig,
the palingenetic cœlomula of the amphioxus (Figs. 80, 81) with the corresponding cenogenetic
form of the amphibia (Figs. 89–90),
and construct the simple diagram that connects the two (Figs. 87, 88). If we imagine the ventral half of
the primitive gut-wall in the amphioxus embryo (Figs. 79–84)
distended with food-yelk, the vesicular cœlom-pouches
(lh) must be pressed together by this, and forced to extend
in the shape of a thin double plate between the gut-wall and
body-wall (Figs. 86, 87). This expansion follows a downward and
forward direction. They are not directly connected with these two
walls. The real unbroken connection between the two middle layers
and the primary germ-layers is found right at the back, in the
region of the primitive mouth (Fig. 87 u). At this important
spot we have the source of embryonic development
(blastocrene), or “zone of growth,” from which
the cœlomation (and also the gastrulation) originally
proceeds.
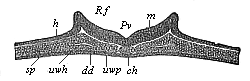 Fig.
92—Transverse section of the chordula-embryo of a
bird (from a hen’s egg at the close of the first day of
incubation). (From Kölliker.) h horn-plate
(ectoderm), m medullary plate, Rf dorsal folds of
same, Pv medullary furrow, ch chorda, uwp
median (inner) part of the middle layer (median wall of the
cœlom-pouches), sp lateral (outer) part of same, or
lateral plates, uwh structure of the body-cavity, dd
gut-gland-layer.
Fig.
92—Transverse section of the chordula-embryo of a
bird (from a hen’s egg at the close of the first day of
incubation). (From Kölliker.) h horn-plate
(ectoderm), m medullary plate, Rf dorsal folds of
same, Pv medullary furrow, ch chorda, uwp
median (inner) part of the middle layer (median wall of the
cœlom-pouches), sp lateral (outer) part of same, or
lateral plates, uwh structure of the body-cavity, dd
gut-gland-layer. |
Hertwig even succeeded in showing, in the cœlomula-embryo
of the water salamander (Triton), between the first
structures of the two middle layers, the relic of the body-cavity,
which is represented in the diagrammatic transitional form (Figs.
87, 88). In sections both through the primitive mouth itself (Fig. 89) and in front of it (Fig. 90) the two
middle layers (pb and vb) diverge from each other,
and disclose the two body-cavities as narrow clefts. At the
primitive-mouth itself (Fig. 90 u) we can penetrate into
them from without. It is only here at the border of the primitive
mouth that we can show the direct transition of the two middle
layers into the two limiting layers or primary germinal layers.
The structure of the chorda also shows the same features in
these cœlomula-embryos of the amphibia (Fig. 91) as in the
amphioxus (Figs. 79–82). It arises from the entodermic
cell-streak, which forms the middle dorsal-line of the primitive
gut, and occupies the space between the flat cœlom-pouches
(Fig. 91 A).
[ 100 ]
While the nervous centre is formed here in the
middle line of the back and separated from the ectoderm as
“medullary tube,” there takes place at the same time,
directly underneath, the severance of the chorda from the entoderm
(Fig. 91 A, B, C). Under the chorda
is formed (out of the ventral entodermic half of the gastrula) the
permanent gut or visceral cavity (enteron) (Fig. 91 B,
dh). This is done by the coalescence, under the chorda in the
median line, of the two dorsal side-borders of the gut-gland-layer
(ik), which were previously separated by the chorda-plate
(Fig. 91 A, ch); these now alone form the clothing of the
visceral cavity (dh) (enteroderm, Fig. 91 C). All
these important modifications take place at first in the fore or
head-part of the embryo, and spread backwards from there; here at
the hinder end, the region of the primitive mouth, the important
border of the mouth (or properistoma) remains for a long
time the source of development or the zone of fresh construction,
in the further building-up of the organism. One has only to compare
carefully the illustrations given (Figs. 85–91) to see that,
as a fact, the cenogenetic cœlomation of the amphibia can be
deduced directly from the palingenetic form of the acrania (Figs.
79–84).
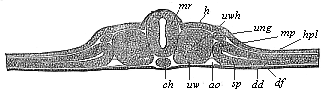
Fig.
93—Transverse section of the vertebrate-embryo of a
bird (from a hen’s egg on the second day of incubation).
(From Kölliker.) h horn-plate, mr
medullary tube, ch chorda, uw primitive segments,
uwh primitive-segment cavity (median relic of the cœlom),
sp lateral cœlom-cleft, hpl skin-fibre-layer,
df gut-fibre-layer, ung primitive-kidney passage,
ao primitive aorta, dd gut-gland-layer. |
The same principle holds good for the amniotes, the reptiles,
birds, and mammals, although in this case the processes of
cœlomation are more modified and more difficult to identify
on account of the colossal accumulation of food-yelk and the
corresponding notable flattening of the germinal disk. However, as
the whole group of the amniotes has been developed at a
comparatively late date from the class of the amphibia, their
cœlomation must also be directly traceable to that of the
latter. This is really possible as a matter of fact; even the older
illustrations showed an essential identity of features. Thus forty
years ago Kölliker gave, in the first edition of his Human
Embryology (1861), some sections of the chicken-embryo, the
features of which could at once be reduced to those already
described and explained in the sense of Hertwig’s
cœlom-theory. A section through the embryo in the hatched
hen’s egg towards the close of the first day of incubation
shows in the middle of the dorsal surface a broad ectodermic
medullary groove (Fig. 92 Rf), and
underneath the middle of the chorda (ch) and at each side of
it a couple of broad mesodermic layers (sp). These enclose a
narrow space or cleft (uwh), which is nothing else than the
structure of the body-cavity. The two layers that enclose
it—the upper parietal layer (hpl) and the lower
visceral layer (df)—are pressed together from without,
but clearly distinguishable. This is even clearer a little later,
when the medullary furrow is closed into the nerve-tube (Fig. 93
mr).
Special importance attaches to the fact that here again the four
secondary germinal layers are already sharply distinct, and easily
separated from each other. There is only one very restricted area
in which they are connected, and actually pass into each other;
this is the region of the primitive mouth, which is contracted in
the amniotes into a dorsal longitudinal cleft, the primitive
groove. Its two lateral lip-borders form the primitive
streak, which has long been recognised as the most important
embryonic source and starting-point of further processes. Sections
through this primitive streak (Figs. 94 and 95) show that the two
primary germinal layers grow at an early stage (in the discoid
gastrula of the chick, a few hours after incubation) into the
primitive
[ 101 ]
streak (x), and that the two middle layers
extend outward from this thickened axial plate (y) to the
right and left between the former. The plates of the
cœlom-layers, the parietal skin-fibre-layer (m) and
the visceral gut-fibre-layer (f), are seen to be still
pressed close together, and only diverge later to form the
body-cavity. Between the inner borders of the two flat
cœlom-pouches lies the chorda (Fig. 95 x), which here
again develops from the middle line of the dorsal wall of the
primitive gut.
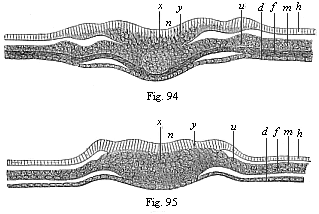
Figs. 94 and
95—Transverse section of the primitive-streak
(primitive mouth) of the chick. Fig. 94 a few hours after the
commencement of incubation, Fig. 95 a little later. (From
Waldeyer.) h horn-plate, n nerve-plate, m
skin-fibre-layer, f gut-fibre-layer, d
gut-gland-layer, y primitive streak or axial plate, in which
all four germinal layers meet, x structure of the chorda,
u region of the later primitive kidneys. |
Cœlomation takes place in the vertebrates in just the same
way as in the birds and reptiles. This was to be expected, as the
characteristic gastrulation of the mammal has descended from that
of the reptiles. In both cases a discoid gastrula with primitive
streak arises from the segmented ovum, a two-layered germinal disk
with long and small hinder primitive mouth. Here again the two
primary germinal layers are only directly connected (Fig. 96
pr) along the primitive streak (at the folding-point of the
blastula), and from this spot (the border of the primitive mouth)
the middle germinal layers (mk) grow out to right and left
between the preceding. In the fine illustration of the
cœlomula of the rabbit which Van Beneden has given us (Fig. 96) one can clearly see that each of the
four secondary germinal layers consists of a single stratum of
cells.
Finally, we must point out, as a fact of the utmost importance
for our anthropogeny and of great general interest, that the
four-layered cœlomula of man has just the same construction
as that of the rabbit (Fig. 96). A vertical section that Count Spee
made through the primitive mouth or streak of a very young human
germinal disk (Fig. 97) clearly shows that here again the four
secondary germ-layers are inseparably connected only at the
primitive streak, and that here also the two flattened
cœlom-pouches (mk) extend outwards to right and left
from the primitive mouth between the outer and inner germinal
layers. In this case, too, the middle germinal layer consists from
the first of two separate strata of cells, the parietal (mp)
and visceral (mv) mesoblasts.
These concordant results of the best recent investigations
(which have been confirmed by the observations of a number of
scientists I have not enumerated) prove the unity of the
vertebrate-stem in point of cœlomation, no less than of
gastrulation. In both respects the invaluable amphioxus—the
sole survivor of the acrania—is found to be the original
model that has preserved for us in palingenetic form by a tenacious
heredity these
[ 102 ]
most important embryonic processes. From this
primary model of construction we can cenogenetically deduce all the
embryonic forms of the other vertebrates, the craniota, by
secondary modifications. My thesis of the universal formation of
the gastrula by folding of the blastula has now been clearly proved
for all the vertebrates; so also has been Hertwig’s thesis of
the origin of the middle germinal layers by the folding of a couple
of cœlom-pouches which appear at the border of the primitive
mouth. Just as the gastræa-theory explains the origin and
identity of the two primary layers, so the cœlom-theory
explains those of the four secondary layers. The point of origin is
always the properistoma, the border of the original primitive mouth
of the gastrula, at which the two primary layers pass directly into
each other.
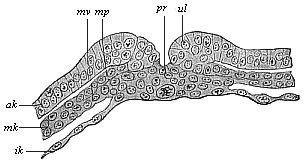
Fig.
96—Transverse section of the primitive groove (or
primitive mouth) of a rabbit. (From Van Beneden.)
pr primitive mouth, ul lips of same (primitive lips),
ak and ik outer and inner germinal layers, mk
middle germinal layer, mp parietal layer, mv visceral
layer of the mesoderm. |
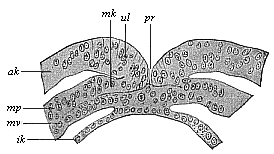
Fig.
97—Transverse section of the primitive mouth (or
groove) of a human embryo (at the cœlomula stage). (From
Count Spee.) pr primitive mouth, ul lips of
same (primitive folds), ak and ik outer and inner
germinal layers, mk middle layer, mp parietal layer,
mv visceral layer of the mesoblasts. |
Moreover, the cœlomula is important as the immediate
source of the chordula, the embryonic reproduction of the ancient,
typical, unarticulated, worm-like form, which has an axial chorda
between the dorsal nerve-tube and the ventral gut-tube. This
instructive chordula (Figs. 83–86) provides a valuable
support of our phylogeny; it indicates the important moment in our
stem-history at which the stem of the chordonia (tunicates and
vertebrates) parted for ever from the divergent stems of the other
metazoa (articulates, echinoderms, and molluscs).
I may express here my opinion, in the form of a
chordæa-theory, that the characteristic chordula-larva of the
chordonia has in reality this great significance—it is the
typical reproduction (preserved by heredity) of the ancient common
stem-form of all the vertebrates and tunicates, the long-extinct
Chordæa. We will return in Chapter XX to these
worm-like ancestors, which stand out as luminous points in the
obscure stem-history of the invertebrate ancestors of our race.
Title and Contents
Glossary
Chapter IX
Chapter XI
Figs. 1–209
Figs. 210–408