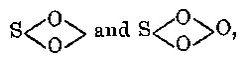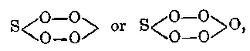*** START OF THE PROJECT GUTENBERG EBOOK 54210 ***
THE
PRINCIPLES OF CHEMISTRY
By D. MENDELÉEFF
TRANSLATED FROM THE RUSSIAN (SIXTH EDITION) BY
GEORGE KAMENSKY, A.R.S.M.
OF THE IMPERIAL MINT, ST PETERSBURG: MEMBER OF THE RUSSIAN PHYSICO-CHEMICAL SOCIETY
EDITED BY
T. A. LAWSON, B.Sc. Ph.D.
EXAMINER IN COAL-TAR PRODUCTS TO THE CITY AND GUILDS OF LONDON INSTITUTE
FELLOW OF THE INSTITUTE OF CHEMISTRY
IN TWO VOLUMES
VOLUME II.
LONGMANS, GREEN, AND CO
39 PATERNOSTER ROW, LONDON
NEW YORK AND BOMBAY
1897
All rights reserved
Table III.
The periodic dependence of the composition of the simplest compounds and properties of the simple bodies upon the
atomic weights of the elements.
Molecular composition of the
higher hydrogen and
metallo-organic compounds |
Atomic weights of the elements |
Composition of the saline compounds, X=Cl |
Peroxides |
Lower hydrogen
compounds |
Simple bodies |
| Sp. gr. |
Sp. vol. |
Melting
point |
| |
|
Br, (NO3), ½O, ½(SO4), OH, (OM)=Z, where M=K |
|
|
|
|
|
| |
|
½Ca, ⅓Al, &c. |
|
|
|
|
|
| E=CH3, C2H5, &c. |
|
Form |
RX |
RX2 |
RX3 |
RX4 |
RX5 |
RX6 |
RX7 |
RX8 |
|
|
|
|
|
| |
|
Oxides |
R2O |
RO |
R2O3 |
RO2 |
R2O5 |
RO3 |
R2O7 |
RO4 |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| [1] |
[2] |
[3] |
[4] |
[5] |
[6] |
|
|
[7] |
[8] |
[9] |
[10] |
[11] |
[12] |
[13] |
[14] |
[15] |
[16] |
[17] |
[18] |
[19] |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
HH |
H |
1,005 |
(mean) |
|
HX or H2O |
|
|
|
|
|
|
|
H2O2 |
— |
*0·05 |
20 |
-250°? |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Li |
7·02 |
(Stas) |
|
LiX |
|
|
|
|
|
|
|
— |
— |
0·59 |
11·9 |
180° |
| |
|
|
|
Be |
9·1 |
(Nilson Pettersson) |
|
— |
BX2 |
|
|
|
|
|
|
— |
BeH |
1·64 |
5·5 |
900°? |
| |
BE3 |
— |
— |
B |
11·0 |
(Ramsay Ashton) |
|
— |
— |
BX3 |
|
|
|
|
|
— |
— |
2·5 |
4·4 |
1,300°? |
| CH4 |
C2H6 |
C2H4 |
C2H2 |
C |
12·0 |
(Roscoe) |
|
— |
CO |
— |
COZ2 |
|
|
|
|
C2O5* |
— |
*1·9 |
6·3 |
2,600°? |
| |
NH3 |
N2H4 |
— |
N |
14·04 |
(Stas) |
|
N2O |
NO |
NOZ |
NO2 |
NO2Z |
|
|
|
N2O6* |
N3H |
*0·6 |
23 |
-203° |
| |
|
OH2 |
— |
O |
16 |
(conventional) |
|
— |
OX2 |
|
|
|
|
|
|
O3 |
— |
*0·9 |
18 |
-230°? |
| |
|
— |
FH |
F |
19·0 |
(Christiansen) |
|
FZ |
— |
|
|
|
|
|
|
— |
— |
?1·0 |
19 |
? |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
NaE |
Na |
23·04 |
(Stas) |
|
NaX |
|
|
|
|
|
|
|
NaO |
Na2H |
0·98 |
23·5 |
96° |
| |
|
MgE2 |
— |
Mg |
24·3 |
(Burton) |
|
— |
MgX2 |
|
|
|
|
|
|
— |
MgH |
1·74 |
14 |
500° |
| |
AlE3 |
— |
— |
Al |
27·1 |
(Mallet) |
|
— |
— |
AlX3 |
|
|
|
|
|
— |
— |
2·6 |
11 |
600° |
| SiH4 |
Si2E6 |
— |
— |
Si |
28·4 |
(Thorpe Young) |
|
— |
— |
— |
SiOZ2 |
|
|
|
|
— |
— |
2·3 |
12 |
1,300°? |
| |
PH3 |
P2H4 |
— |
P |
31·0 |
(v. d. Plaats) |
|
— |
— |
PX3 |
— |
POZ3 |
|
|
|
— |
P2H |
2·2 |
14 |
44° |
| |
|
SH2 |
— |
S |
32·06 |
(Stas) |
|
— |
SX2 |
— |
SOZ2 |
— |
SO2Z2 |
|
|
S2O7 |
— |
2·07 |
15 |
114° |
| |
|
|
ClH |
Cl |
35·45 |
(Stas) |
|
ClZ |
— |
ClOZ |
— |
ClO2Z |
— |
ClO3Z |
|
— |
— |
*1·3 |
27 |
-75° |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
K |
39·15 |
(Stas) |
|
KX |
|
|
|
|
|
|
|
KO2 |
K2H |
0·87 |
45 |
58° |
| |
|
|
|
Ca |
40·0 |
(Dumas) |
|
— |
CaX2 |
|
|
|
|
|
|
CaO2 |
CaH |
1·56 |
26 |
800° |
| |
|
|
|
Sc |
44·0 |
(Nilson) |
|
— |
— |
ScX3 |
|
|
|
|
|
— |
— |
?2·5 |
?18 |
1,200°? |
| |
|
|
|
Ti |
48·1 |
(Thorpe) |
|
— |
TiX2 |
TiX3 |
TiX4 |
|
|
|
|
TiO3 |
— |
3·6 |
13 |
2,500°? |
| |
|
|
|
V |
51·2 |
(Roscoe) |
|
— |
VO |
VOX |
— |
VOZ3 |
— |
|
|
— |
— |
5·5 |
9 |
3,000°? |
| |
|
|
|
Cr |
52·1 |
(Rawson) |
|
— |
CrX2 |
CrX3 |
CrO2 |
— |
CrO2Z2 |
|
|
Cr2O7 |
— |
6·7 |
7·7 |
2,000°? |
| |
|
|
|
Mn |
55·1 |
(Marignac) |
|
— |
MnX2 |
MnX3 |
MnO2 |
— |
MnO2Z2 |
MnO3Z |
|
— |
— |
7·5 |
7·3 |
1,500° |
| |
|
|
|
Fe |
56·0 |
(Dumas) |
|
— |
FeX2 |
FeX3 |
— |
— |
FeO2Z2 |
|
|
— |
FenH* |
7·8 |
7·2 |
1,450° |
| |
|
|
|
Co |
58·9 |
(Zimmermann) |
|
— |
CoX2 |
CoX3 |
CoO2 |
|
|
|
|
— |
— |
8·6 |
6·8 |
1,400° |
| |
|
|
|
Ni |
59·4 |
(Winkler) |
|
— |
NiX2 |
NiX3 |
|
|
|
|
|
— |
NinH |
8·7 |
6·8 |
1,350° |
| |
|
|
|
Cu |
63·6 |
(Richards) |
|
CuX |
CuX2 |
|
|
|
|
|
|
Cu2O5* |
CuH |
8·8 |
7·2 |
1,054° |
| |
|
ZnE2 |
— |
Zn |
65·3 |
(Marignac) |
|
— |
ZnX2 |
|
|
|
|
|
|
ZnO2 |
— |
7·1 |
9·2 |
418° |
| |
GaE3 |
— |
— |
Ga |
69·9 |
(Boisbaudran) |
|
— |
— |
GaX3 |
|
|
|
|
|
— |
— |
5·96 |
11·7 |
30° |
| GeE4 |
— |
— |
— |
Ge |
72·3 |
(Winkler) |
|
— |
GaX2 |
— |
GaX4 |
|
|
|
|
— |
— |
5·47 |
13·2 |
900° |
| |
AsH3 |
— |
— |
As |
75·0 |
(Dumas) |
|
— |
AsS |
AsX3 |
AsS2 |
AsO2Z |
|
|
|
— |
As4H* |
5·65 |
13·3 |
500° |
| |
|
SeH2 |
— |
Se |
79·0[A] |
(Pettersson) |
|
— |
— |
— |
SeOZ2 |
— |
SeO2Z2 |
|
|
— |
— |
4·8 |
16 |
217° |
| |
|
|
BrH |
Br |
79·95 |
(Stas) |
|
BrZ |
— |
BrOZ |
— |
BrO2Z |
— |
BrO3Z |
|
— |
— |
3·1 |
26 |
-7° |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Rb |
85·5 |
(Godeffroy) |
|
RbX |
|
|
|
|
|
|
|
RbO |
Rb2H* |
1·5 |
57 |
39° |
| |
|
|
|
Sr |
87·6 |
(Dumas) |
|
— |
SrX2 |
|
|
|
|
|
|
SrO2 |
SrH |
2·5 |
35 |
600°? |
| |
|
|
|
Y |
89 |
(Clève) |
|
— |
— |
YX3 |
|
|
|
|
|
— |
— |
*3·4 |
*26 |
1,000°? |
| |
|
|
|
Zr |
90·6 |
(Bailey) |
|
— |
— |
— |
ZrX4 |
|
|
|
|
— |
Zr4nH* |
4·1 |
2·2 |
1,500°? |
| |
|
|
|
Nb |
94 |
(Marignac) |
|
— |
— |
NbX3 |
— |
NbO2Z |
|
|
|
— |
NbnH* |
7·1 |
13 |
1,800°? |
| |
|
|
|
Mo |
96·1 |
(Maas) |
|
— |
— |
MoX3 |
MoX4 |
— |
MoO2Z2 |
|
|
Mo2O7 |
— |
8·6 |
11 |
2,200°? |
| Unknown metal (eka-manganese, Em = 99). |
EmO3Z |
|
— |
— |
— |
— |
— |
| |
|
|
|
Ru |
101·7 |
(Joly) |
|
— |
RuX2 |
RuX3 |
RuX4 |
— |
RuO2Z2 |
— |
RuO4 |
— |
RunH* |
12·2 |
8·4 |
2,000°? |
| |
|
|
|
Rh |
102·7 |
(Seubert) |
|
— |
RhX2 |
RhX3 |
RhX4 |
— |
RhO2Z2 |
|
|
— |
RhnH* |
12·1 |
8·6 |
1,900°? |
| |
|
|
|
Pd |
106·4 |
(Keller Smith) |
|
PdX |
PdX2 |
— |
PdX4 |
|
|
|
|
— |
Pd2H |
11·4 |
8·3 |
1,500° |
| |
|
|
|
Ag |
107·92 |
(Stas) |
|
AgX |
|
|
|
|
|
|
|
AgO |
— |
10·5 |
10·3 |
950° |
| |
|
CdE2 |
— |
Cd |
112·1 |
(Lorimer Smith) |
|
— |
CdX2 |
|
|
|
|
|
|
CdO2 |
— |
8·6 |
13 |
320° |
| |
InE3 |
— |
— |
In |
113·6 |
(Winkler) |
|
— |
InX2 |
InX3 |
|
|
|
|
|
— |
— |
7·4 |
14 |
176° |
| SnE4 |
— |
— |
— |
Sn |
119·1 |
(Classen) |
|
— |
SnX2 |
— |
SnX4 |
|
|
|
|
SnO3 |
— |
7·2 |
16 |
232° |
| |
SbH3 |
— |
— |
Sb |
120·4 |
(Schneider) |
|
— |
— |
SbX3 |
— |
SbO2Z |
|
|
|
— |
— |
6·7 |
18 |
432° |
| |
|
TeH2 |
— |
Te |
125·1 |
(Brauner) |
|
— |
— |
— |
TeOZ2 |
|
|
|
|
— |
— |
6·4 |
20 |
455° |
| |
|
|
IH |
I |
126·85 |
(Stas) |
|
IZ |
— |
IZ3 |
— |
IO2Z |
— |
IO3Z |
|
— |
— |
4·9 |
26 |
114° |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Cs |
132·7 |
(Godeffroy) |
|
CsX |
|
|
|
|
|
|
|
— |
Cs2H* |
2·37 |
56 |
27° |
| |
|
|
|
Ba |
137·4 |
(Richards) |
|
— |
BaX2 |
|
|
|
|
|
|
BaO2 |
BaH |
3·76 |
36 |
? |
| |
|
|
|
La |
138·2 |
(Brauner) |
|
— |
— |
LaX3 |
|
|
|
|
|
— |
— |
6·1 |
23 |
? |
| |
|
|
|
Ce |
140·2 |
(Brauner) |
|
— |
— |
CeX3 |
CeX4 |
|
|
|
|
— |
— |
6·6 |
21 |
700°? |
| Little known Di = 142.1 and Yb = 173.2, and over 15 unknown elements. |
|
|
|
|
|
|
| |
|
|
|
Ta |
182·7 |
(Marignac) |
|
— |
— |
— |
— |
TaO2Z |
|
|
|
— |
TanH* |
10·4 |
18 |
? |
| |
|
|
|
W |
184·0 |
(Waddel) |
|
— |
— |
— |
WX4 |
— |
WO2Z2 |
|
|
W2O7 |
— |
19·1 |
9·6 |
2,600° |
| Unknown element. |
|
|
|
|
|
|
| |
|
|
|
Os |
191·6 |
(Seubert) |
|
— |
— |
OsX3 |
OsX4 |
— |
OsO2Z2 |
— |
OsO4 |
— |
— |
22·5 |
8·5 |
2,700°? |
| |
|
|
|
Ir |
193·3 |
(Joly) |
|
— |
— |
IrX3 |
IrX4 |
— |
IrO2Z2 |
|
|
— |
IrnH* |
22·4 |
8·6 |
2,000° |
| |
|
|
|
Pt |
196·0 |
(Dittmar McArthur) |
|
— |
PtX2 |
— |
PtX4 |
|
|
|
|
— |
PtnH* |
21·4 |
9·2 |
1,775° |
| |
|
|
|
Au |
197·5 |
(Dittmar McArthur) |
|
AuX |
— |
AuX3 |
|
|
|
|
|
— |
— |
19·3 |
10 |
1,045° |
| |
|
HgE2 |
— |
Hg |
200·5 |
(Erdmann Mar.) |
|
HgX |
HgX2 |
|
|
|
|
|
|
— |
— |
13·6 |
15 |
-39° |
| |
TlE3 |
— |
— |
Tl |
204·1 |
(Crookes) |
|
TlX |
— |
TlX3 |
|
|
|
|
|
— |
— |
11·8 |
17 |
294° |
| PbE4 |
— |
— |
— |
Pb |
206·90 |
(Stas) |
|
— |
PbX2 |
— |
PbOZ2 |
|
|
|
|
— |
— |
11·3 |
18 |
328° |
| |
BiE3 |
— |
— |
Bi |
208·9 |
(Classen) |
|
— |
— |
BiX3 |
— |
BiO2 |
|
|
|
— |
— |
9·8 |
21 |
269° |
| Five unknown elements. |
|
|
|
|
|
|
| |
|
|
|
Th |
232·4 |
(Krüss Nilson) |
|
— |
— |
— |
ThX4 |
|
|
|
|
— |
— |
11·1 |
21 |
? |
| Unknown element. |
|
|
|
|
|
|
| |
|
|
|
U |
239·3 |
(Zimmermann) |
|
— |
— |
— |
UO2 |
— |
UO2X2 |
— |
UO4 |
— |
— |
18·7 |
13 |
2,400°? |
Columns 1, 2, 3, and 4 give the molecular composition of the hydrogen and metallo-organic compounds, exhibiting the most characteristic forms
assumed by the elements. The first column contains only those which correspond to the form RX4, the second column those of the form RX3, the third
of the form RX2, and the fourth of the form RX, so that the periodicity stands out clearly (see Column 16).
Column 5 contains the symbols of all the more or less well-known elements, placed according to the order of the magnitude of their atomic weights.
Column 6 contains the atomic weights of the elements according to the most trustworthy determinations. The names of the investigators are
given in parenthesis. The atomic weight of oxygen, taken as 16, forms the basis upon which these atomic weights were calculated. Some of these
have been recalculated by me on the basis of Stas's most trustworthy data (see Chapter XXIV. and the numbers given by Stas in the table, where they
are taken according to van der Plaats and Thomsen's calculations).
Columns 7–14 contain the composition of the saline compounds of the elements, placed according to their forms, RX, RX2 to RX8 (in the 14th
column). If the element R has a metallic character like H, Li, Be, &c., then X represents Cl, NO3, ½ SO4, &c., haloid radicles, or (OH) if a perfect
hydrate is formed (alkali, aqueous base), or ½ O, ½ S, &c. when an anhydrous oxide, sulphide, &c. is formed. For instance, NaCl, Mg(NO3)2, Al2(SO4)3,
correspond to NaX, MgX2, and AlX3; so also Na(OH), Mg(OH)2, Al(OH)3, Na2O, MgO, Al2O3, &c. But if the element, like C or N, be of a metalloid
or acid character, X must be regarded as (OH) in the formation of hydrates; (OM) in the formation of salts, where M is the equivalent of a
metal, ½ O in the formation of an anhydride, and Cl in the formation of a chloranhydride; and in this case (i.e. in the acid compounds) Z is put in
the place of X; for example, the formulæ COZ2, NO2Z, MNO2Z, FeO2Z2, and IZ3 correspond to CO(NaO)2 = Na2CO3, COCl2, CO2, NO2(NaO) = NaNO3,
NO2Cl, NO2(OH) = HNO3; MnO3(OK) = KMnO4, ICl, &c.
The 15th column gives the compositions of the peroxides of the elements, taking them as anhydrous. An asterisk (*) is attached to those of which
the composition has not been well established, and a dash (—) shows that for a given element no peroxides have yet been obtained. The peroxides
contain more oxygen than the higher saline oxides of the same elements, are powerfully oxidising, and easily give peroxide of hydrogen. This latter
circumstance necessitates their being referred to the type of peroxide of hydrogen, if bases and acids are referred to the type of water (see Chapter XV.,
Note 7 and 11 bis).
The 16th column gives the composition of the lower hydrogen compounds like N3H and Na2H. They may often be regarded as alloys of hydrogen,
which is frequently disengaged by them at a comparatively moderate temperature. They differ greatly in their nature from the hydrogen compounds
given in columns 1–4 (see Note 12).
Column 17 gives the specific gravity of the elements in a solid and a liquid state. An asterisk (*) is placed by those which can either only be
assumed from analogy (for example, the sp. gr. of fluorine and hydrogen, which have not been obtained in a liquid state), or which vary very rapidly
with a variation of temperature and pressure (like oxygen and nitrogen), or physical state (for instance, carbon in passing from the state of charcoal
to graphite and diamond). But as the sp. gr. in general varies with the temperature, mechanical condition, &c., the figures given, although chosen
from the most trustworthy sources, can only be regarded as approximate, and not as absolutely true. They clearly show a certain periodicity; for
instance, the sp. gr. diminishes from Al on both sides (Al, Mg, Na, with decreasing atomic weight; and Al, Si, P, S, Cl, with increasing atomic weight,
it also diminishes on both sides from Cu, Ru, and Os.)
The same remarks refer to the figures in the 18th column, which gives the so-called atomic volumes of the simple bodies, or the quotient of their
atomic weight and specific gravity. For Na, K, Rb, and Cs the atomic volume is greatest among the neighbouring elements. For Ni, Pd, and Os it is
least, and this indicates the periodicity of this property of the simple bodies.
The last (19th) column gives the melting points of the simple bodies. Here also a periodicity is seen, i.e. a maximum and minimum value
between which there are intermediate values, as we see, for instance, in the series Cl, K, Ca, Sc, and Ti, or in the series Cr, Mn, Fe, Co, Ni, Cu, Zn,
Ga, and Ge.
[1]
PRINCIPLES OF CHEMISTRY
CHAPTER XV
THE GROUPING OF THE ELEMENTS AND THE PERIODIC LAW
It is seen from the examples given in the preceding chapters that the
sum of the data concerning the chemical transformations proper to the
elements (for instance, with respect to the formation of acids, salts,
and other compounds having definite properties) is insufficient for
accurately determining the relationship of the elements, inasmuch
as this may be many-sided. Thus, lithium and barium are in
some respects analogous to sodium and potassium, and in others to
magnesium and calcium. It is evident, therefore, that for a complete
judgment it is necessary to have, not only qualitative, but also
quantitative, exact and measurable, data. When a property can be measured
it ceases to be vague, and becomes quantitative instead of
merely qualitative.
Among these measurable properties of the elements, or of their
corresponding compounds, are: (a) isomorphism, or the analogy of
crystalline forms; and, connected with it, the power to form crystalline
mixtures which are isomorphous; (b) the relation of the volumes of
analogous compounds of the elements; (c) the composition of their
saline compounds; and (d) the relation of the atomic weights of the
elements. In this chapter we shall briefly consider these four aspects
of the matter, which are exceedingly important for a natural and
fruitful grouping of the elements, facilitating, not only a general
acquaintance with them, but also their detailed study.
Historically the first, and an important and convincing, method for
finding a relationship between the compounds of two different elements
is by isomorphism. This conception was introduced into chemistry
by Mitscherlich (in 1820), who demonstrated that the corresponding
salts of arsenic acid, H3AsO4, and phosphoric acid, H3PO4, crystallise[2]
with an equal quantity of water, show an exceedingly close resemblance
in crystalline form (as regards the angles of their faces and axes), and
are able to crystallise together from solutions, forming crystals containing
a mixture of the isomorphous compounds. Isomorphous substances
are those which, with an equal number of atoms in their
molecules, present an analogy in their chemical reactions, a close
resemblance in their properties, and a similar or very nearly similar
crystalline form: they often contain certain elements in common, from
which it is to be concluded that the remaining elements (as in the
preceding example of As and P) are analogous to each other. And
inasmuch as crystalline forms are capable of exact measurement,
the external form, or the relation of the molecules which causes
their grouping into a crystalline form, is evidently as great a help in
judging of the internal forces acting between the atoms as a comparison
of reactions, vapour densities, and other like relations. We have
already seen examples of this in the preceding pages.[1] It will be
sufficient to call to mind that the compounds of the alkali metals
with the halogens RX, in a crystalline form, all belong to the cubic
system and crystallise in octahedra or cubes—for example, sodium
chloride, potassium chloride, potassium iodide, rubidium chloride, &c.
The nitrates of rubidium and cæsium appear in anhydrous crystals of
the same form as potassium nitrate. The carbonates of the metals of
the alkaline earths are isomorphous with calcium carbonate—that is,
they either appear in forms like calc spar or in the rhombic system
in crystals analogous to aragonite.[1 bis] Furthermore, sodium nitrate
crystallises in rhombohedra, closely resembling the rhombohedra of calc
spar (calcium carbonate), CaCO3, whilst potassium nitrate appears in the
same form as aragonite, CaCO3, and the number of atoms in both kinds
of salts is the same: they all contain one atom of a metal (K, Na, Ca), one
atom of a non-metal (C, N), and three atoms of oxygen. The analogy
of form evidently coincides with an analogy of atomic composition.
But, as we have learnt from the previous description of these salts, there
is not any close resemblance in their properties. It is evident that calcium
carbonate approaches more nearly to magnesium carbonate than to sodium
nitrate, although their crystalline forms are all equally alike. Isomorphous[3]
substances which are perfectly analogous to each other are not
only characterised by a close resemblance of form (homeomorphism), but
also by the faculty of entering into analogous reactions, which is not the
case with RNO3 and RCO3. The most important and direct method of
recognising perfect isomorphism—that is, the absolute analogy of two
compounds—is given by that property of analogous compounds of
separating from solutions in homogeneous crystals, containing the
most varied proportions of the analogous substances which enter
into their composition. These quantities do not seem to be in
dependence on the molecular or atomic weights, and if they are
governed by any laws they must be analogous to those which apply to
indefinite chemical compounds.[2] This will be clear from the following
examples. Potassium chloride and potassium nitrate are not
isomorphous with each other, and are in an atomic sense composed
in a different manner. If these salts be mixed in a solution and
the solution be evaporated, independent crystals of the two salts will
separate, each in that crystalline form which is proper to it. The
crystals will not contain a mixture of the two salts. But if we
mix the solutions of two isomorphous salts together, then, under
certain circumstances, crystals will be obtained which contain both
these substances. However, this cannot be taken as an absolute rule,
for if we take a solution saturated at a high temperature with a
mixture of potassium and sodium chlorides, then on evaporation sodium
chloride only will separate, and on cooling only potassium chloride.[4]
The first will contain very little potassium chloride, and the latter
very little sodium chloride.[3] But if we take, for example, a mixture
of solutions of magnesium sulphate and zinc sulphate, they cannot
be separated from each other by evaporating the mixture, notwithstanding
the rather considerable difference in the solubility of these
salts. Again, the isomorphous salts, magnesium carbonate, and calcium
carbonate are found together—that is, in one crystal—in nature.
The angle of the rhombohedron of these magnesia-lime spars is intermediate
between the angles proper to the two spars individually (for calcium
carbonate, the angle of the rhombohedron is 105° 8′; magnesium
carbonate, 107° 30′; CaMg(CO3)2, 106° 10′). Certain of these isomorphous
mixtures of calc and magnesia spars appear in well-formed crystals,
and in this case there not unfrequently exists a simple molecular proportion
of strictly definite chemical combination between the component
salts—for instance, CaCO3,MgCO3—whilst in other cases, especially in
the absence of distinct crystallisation (in dolomites), no such simple
molecular proportion is observable: this is also the case in many
artificially prepared isomorphous mixtures. The microscopical and
crystallo-optical researches of Professor Inostrantzoff and others
show that in many cases there is really a mechanical, although microscopically
minute, juxtaposition in one whole of the heterogeneous
crystals of calcium carbonate (double refracting) and of the compound
CaMgC2O6. If we suppose the adjacent parts to be microscopically
small (on the basis of the researches of Mallard, Weruboff, and others),
we obtain an idea of isomorphous mixtures. A formula of the following
kind is given to isomorphous mixtures: for instance, for spars,
RCO3, where R = Mg, Ca, and where it may be Fe,Mn …, &c. This
means that the Ca is partially replaced by Mg or another metal.
Alums form a common example of the separation of isomorphous[5]
mixtures from solutions. They are double sulphates (or seleniates)
of alumina (or oxides isomorphous with it) and the alkalis, which
crystallise in well-formed crystals. If aluminium sulphate be mixed
with potassium sulphate, an alum separates, having the composition
KAlS2O8,12H2O. If sodium sulphate or ammonium sulphate, or
rubidium (or thallium) sulphate be used, we obtain alums having the
composition RAlS2O8,12H2O. Not only do they all crystallise in the
cubic system, but they also contain an equal atomic quantity of water
of crystallisation (12H2O). Besides which, if we mix solutions of the
potassium and ammonium (NH4AlS2O8,12H2O) alums together, then
the crystals which separate will contain various proportions of the
alkalis taken, and separate crystals of the alums of one or the other
kind will not be obtained, but each separate crystal will contain both
potassium and ammonium. Nor is this all; if we take a crystal of a
potassium alum and immerse it in a solution capable of yielding
ammonia alum, the crystal of the potash alum will continue to
grow and increase in size in this solution—that is, a layer of the
ammonia or other alum will deposit itself upon the planes bounding
the crystal of the potash alum. This is very distinctly seen if a colourless
crystal of a common alum be immersed in a saturated violet solution
of chrome alum, KCrS2O8,12H2O, which then deposits itself in a
violet layer over the colourless crystal of the alumina alum, as was
observed even before Mitscherlich noticed it. If this crystal be then
immersed in a solution of an alumina alum, a layer of this salt will
form over the layer of chrome alum, so that one alum is able to incite
the growth of the other. If the deposition proceed simultaneously, the
resultant intermixture may be minute and inseparable, but its nature is
understood from the preceding experiments; the attractive force of
crystallisation of isomorphous substances is so nearly equal that the
attractive power of an isomorphous substance induces a crystalline superstructure
exactly the same as would be produced by the attractive force
of like crystalline particles. From this it is evident that one isomorphous
substance may induce the crystallisation[4] of another. Such a phenomenon
explains, on the one hand, the aggregation of different isomorphous
substances in one crystal, whilst, on the other hand, it serves as a most
exact indication of the nearness both of the molecular composition of
isomorphous substances and of those forces which are proper to the
elements which distinguish the isomorphous substances. Thus, for
example, ferrous sulphate or green vitriol crystallises in the monoclinic[6]
system and contains seven molecules of water, FeSO4,7H2O, whilst
copper vitriol crystallises with five molecules of water in the triclinic
system, CuSO4,5H2O; nevertheless, it may be easily proved that both
salts are perfectly isomorphous; that they are able to appear in identically
the same forms and with an equal molecular amount of water.
For instance, Marignac, by evaporating a mixture of sulphuric acid
and ferrous sulphate under the receiver of an air-pump, first obtained
crystals of the hepta-hydrated salt, and then of the penta-hydrated
salt FeSO4,5H2O, which were perfectly similar to the crystals of copper
sulphate. Furthermore, Lecoq de Boisbaudran, by immersing crystals
of FeSO4,7H2O in a supersaturated solution of copper sulphate, caused
the latter to deposit in the same form as ferrous sulphate, in crystals
of the monoclinic system, CuSO4,7H2O.
Hence it is evident that isomorphism—that is, the analogy of forms
and the property of inducing crystallisation—may serve as a means for
the discovery of analogies in molecular composition. We will take an
example in order to render this clear. If, instead of aluminium sulphate,
we add magnesium sulphate to potassium sulphate, then, on
evaporating the solution, the double salt K2MgS2O8,6H2O (Chapter
XIV., Note 28) separates instead of an alum, and the ratio of
the component parts (in alums one atom of potassium per 2SO4, and
here two atoms) and the amount of water of crystallisation (in alums
12, and here 6 equivalents per 2SO4) are quite different; nor is this
double salt in any way isomorphous with the alums, nor capable of
forming an isomorphous crystalline mixture with them, nor does the
one salt provoke the crystallisation of the other. From this we
must conclude that although alumina and magnesia, or aluminium
and magnesium, resemble each other, they are not isomorphous,
and that although they give partially similar double salts, these salts
are not analogous to each other. And this is expressed in their
chemical formulæ by the fact that the number of atoms in alumina or
aluminium oxide, Al2O3, is different from the number in magnesia, MgO.
Aluminium is trivalent and magnesium bivalent. Thus, having obtained
a double salt from a given metal, it is possible to judge of the analogy
of the given metal with aluminium or with magnesium, or of the
absence of such an analogy, from the composition and form of this
salt. Thus zinc, for example, does not form alums, but forms a double
salt with potassium sulphate, which has a composition exactly like that
of the corresponding salt of magnesium. It is often possible to distinguish
the bivalent metals analogous to magnesium or calcium from
the trivalent metals, like aluminium, by such a method. Furthermore,
the specific heat and vapour density serve as guides. There are[7]
also indirect proofs. Thus iron gives ferrous compounds, FeX2, which
are isomorphous with the compounds of magnesium, and ferric
compounds, FeX3, which are isomorphous with the compounds of
aluminium; in this instance the relative composition is directly
determined by analysis, because, for a given amount of iron, FeCl2
only contains two-thirds of the amount of chlorine which occurs in
FeCl3, and the composition of the corresponding oxygen compounds,
i.e. of ferrous oxide, FeO, and ferric oxide, Fe2O3, clearly indicates
the analogy of the ferrous oxide with MgO and of the ferric oxide
with Al2O3.
Thus in the building up of similar molecules in crystalline forms we
see one of the numerous means for judging of the internal world of
molecules and atoms, and one of the weapons for conquests in the
invisible world of molecular mechanics which forms the main object of
physico-chemical knowledge. This method[5] has more than once been[8]
employed for discovering the analogy of elements and of their compounds;
and as crystals are measurable, and the capacity to form[9]
crystalline mixtures can be experimentally verified, this method is a
numerical and measurable one, and in no sense arbitrary.
[10]
The regularity and simplicity expressed by the exact laws of crystalline
form repeat themselves in the aggregation of the atoms to form
molecules. Here, as there, there are but few forms which are essentially
different, and their apparent diversity reduces itself to a few
fundamental differences of type. There the molecules aggregate
themselves into crystalline forms; here, the atoms aggregate themselves
into molecular forms or into the types of compounds. In both
cases the fundamental crystalline or molecular forms are liable to
variations, conjunctions, and combinations. If we know that potassium
gives compounds of the fundamental type KX, where X is a univalent
element (which combines with one atom of hydrogen, and is, according
to the law of substitution, able to replace it), then we know the composition
of its compounds: K2O, KHO, KCl, NH2K, KNO3, K2SO4,
KHSO4, K2Mg(SO4)2,6H2O, &c. All the possible derivative crystalline
forms are not known. So also all the atomic combinations are not
known for every element. Thus in the case of potassium, KCH3, K3P,[11]
K2Pt, and other like compounds which exist for hydrogen or chlorine,
are unknown.
Only a few fundamental types exist for the building up of atoms
into molecules, and the majority of them are already known to us. If
X stand for a univalent element, and R for an element combined with
it, then eight atomic types may be observed:—
RX, RX2, RX3, RX4, RX5, RX6, RX7, RX8.
Let X be chlorine or hydrogen. Then as examples of the first type
we have: H2, Cl2, HCl, KCl, NaCl, &c. The compounds of oxygen or
calcium may serve as examples of the type RX2: OH2, OCl2, OHCl,
CaO, Ca(OH)2, CaCl2, &c. For the third type RX3 we know the
representative NH3 and the corresponding compounds N2O3, NO(OH),
NO(OK), PCl3, P2O3, PH3, SbH3, Sb2O3, B2O3, BCl3, Al2O3, &c.
The type RX4 is known among the hydrogen compounds. Marsh gas,
CH4, and its corresponding saturated hydrocarbons, CnH2n+2, are the
best representatives. Also CH3Cl, CCl4, SiCl4, SnCl4, SnO2, CO2, SiO2,
and a whole series of other compounds come under this class. The type
RX5 is also already familiar to us, but there are no purely hydrogen
compounds among its representatives. Sal-ammoniac, NH4Cl, and
the corresponding NH4(OH), NO2(OH), ClO2(OK), as well as PCl5,
POCl3, &c., are representatives of this type. In the higher types also
there are no hydrogen compounds, but in the type RX6 there is
the chlorine compound WCl6. However, there are many oxygen compounds,
and among them SO3 is the best known representative. To this
class also belong SO2(OH)2, SO2Cl2, SO2(OH)Cl, CrO3, &c., all of an
acid character. Of the higher types there are in general only oxygen
and acid representatives. The type RX7 we know in perchloric acid,
ClO3(OH), and potassium permanganate, MnO3(OK), is also a member.
The type RX8 in a free state is very rare; osmic anhydride, OsO4, is
the best known representative of it.[6]
[12]
The four lower types RX, RX2, RX3, and RX4 are met with in
compounds of the elements R with chlorine and oxygen, and also in
their compounds with hydrogen, whilst the four higher types only
appear for such acid compounds as are formed by chlorine, oxygen, and
similar elements.
Among the oxygen compounds the saline oxides which are capable
of forming salts either through the function of a base or through the
function of an acid anhydride attract the greatest interest in every
respect. Certain elements, like calcium and magnesium, only give one
saline oxide—for example, MgO, corresponding with the type MgX2.
But the majority of the elements appear in several such forms. Thus
copper gives CuX and CuX2, or Cu2O and CuO. If an element R
gives a higher type RXn, then there often also exist, as if by symmetry,
lower types, RXn-2, RXn-4, and in general such types as differ from
RXn by an even number of X. Thus in the case of sulphur the
types SX2, SX4, and SX6 are known—for example SH2, SO2, and
SO3. The last type is the highest, SX6. The types SX5 and SX3 do
not exist. But even and uneven types sometimes appear for one
and the same element. Thus the types RX and RX2 are known for
copper and mercury.
Among the saline oxides only the eight types enumerated below
are known to exist. They determine the possible formulæ of the compounds
of the elements, if it be taken into consideration that an
element which gives a certain type of combination may also give
lower types. For this reason the rare type of the suboxides or
quaternary oxides R4O (for instance, Ag4O, Ag2Cl) is not characteristic;[13]
it is always accompanied by one of the higher grades of oxidation,
and the compounds of this type are distinguished by their great
chemical instability, and split up into an element and the higher compound
(for instance, Ag4O = 2Ag + Ag2O). Many elements, moreover,
form transition oxides whose composition is intermediate, which are
able, like N2O4, to split up into the lower and higher oxides. Thus
iron gives magnetic oxide, Fe3O4, which is in all respects (by its reactions)
a compound of the suboxide FeO with the oxide Fe2O3. The
independent and more or less stable saline compounds correspond with
the following eight types :—
R2O; salts RX, hydroxides ROH. Generally basic like K2O, Na2O,
Hg2O, Ag2O, Cu2O; if there are acid oxides of this composition they
are very rare, are only formed by distinctly acid elements, and even
then have only feeble acid properties; for example, Cl2O and N2O.
R2O2 or RO; salts RX2, hydroxides R(OH)2. The most simple basic
salts R2OX2 or R(OH)X; for instance, the chloride Zn2OCl2; also
an almost exclusively basic type; but the basic properties are more
feebly developed than in the preceding type. For example, CaO,
MgO, BaO, PbO, FeO, MnO, &c.
R2O3; salts RX3, hydroxides R(OH)3, RO(OH), the most simple basic
salts ROX, R(OH)X3. The bases are feeble, like Al2O3, Fe2O3,
Tl2O3, Sb2O3. The acid properties are also feebly developed; for
instance, in B2O3; but with the non-metals the properties of acids
are already clear; for instance, P2O3, P(OH)3.
R2O4 or RO2; salts RX4 or ROX2, hydroxides R(OH)4, RO(OH)2.
Rarely bases (feeble), like ZrO2, PtO2; more often acid oxides;
but the acid properties are in general feeble, as in CO2, SO2,
SnO2. Many intermediate oxides appear in this and the preceding
and following types.
R2O5; salts principally of the types ROX3, RO2X, RO(OH)3,
RO2(OH), rarely RX5. The basic character (X, a halogen,
simple or complex; for instance, NO3, Cl, &c.) is feeble; the acid
character predominates, as is seen in N2O5, P2O5, Cl2O5; then
X = OH, OK, &c., for example NO2(OK).
R2O6 or RO3; salts and hydroxides generally of the type RO2X2,
RO2(OH)2. Oxides of an acid character, as SO3, CrO3, MnO3.
Basic properties rare and feebly developed as in UO3.
R2O7; salts of the form RO3X, RO3(OH), acid oxides; for instance,
Cl2O7, Mn2O7. Basic properties as feebly developed as the acid
properties in the oxides R2O.
R2O8 or RO4. A very rare type, and only known in OsO4 and
RuO4.
[14]
It is evident from the circumstance that in all the higher types
the acid hydroxides (for example, HClO4, H2SO4, H3PO4) and salts
with a single atom of one element contain, like the higher saline
type RO4, not more than four atoms of oxygen; that the formation
of the saline oxides is governed by a certain common principle which
is best looked for in the fundamental properties of oxygen, and in
general of the most simple compounds. The hydrate of the oxide
RO2 is of the higher type RO22H2O = RH4O4 = R(HO)4. Such,
for example, is the hydrate of silica and the salts (orthosilicates)
corresponding with it, Si(MO)4. The oxide R2O5, corresponds with
the hydrate R2O53H2O = 2RH3O4 = 2RO(OH)3. Such is orthophosphoric
acid, PH3O3. The hydrate of the oxide RO3 is
RO3H2O = RH2O4 = RO2(OH)2—for instance, sulphuric acid. The
hydrate corresponding to R2O7 is evidently RHO = RO3(OH)—for
example, perchloric acid. Here, besides containing O4, it
must further be remarked that the amount of hydrogen in the hydrate
is equal to the amount of hydrogen in the hydrogen compound. Thus
silicon gives SiH4 and SiH4O4, phosphorus PH3 and PH3O4, sulphur
SH2 and SH2O4, chlorine ClH and ClHO4. This, if it does not
explain, at least connects in a harmonious and general system the
fact that the elements are capable of combining with a greater amount of
oxygen, the less the amount of hydrogen which they are able to retain.
In this the key to the comprehension of all further deductions must be
looked for, and we will therefore formulate this rule in general terms.
An element R gives a hydrogen compound RHn, the hydrate of its
higher oxide will be RHnO4, and therefore the higher oxide will contain
2RHnO4 - nH2O = R2O8 - n. For example, chlorine gives ClH, hydrate
ClHO4, and the higher oxide Cl2O7. Carbon gives CH4 and CO2.
So also, SiO2 and SiH4 are the higher compounds of silicon with
hydrogen and oxygen, like CO2 and CH4. Here the amounts of oxygen
and hydrogen are equivalent. Nitrogen combines with a large amount
of oxygen, forming N2O5, but, on the other hand, with a small quantity
of hydrogen in NH3. The sum of the equivalents of hydrogen and
oxygen, occurring in combination with an atom of nitrogen, is, as
always in the higher types, equal to eight. It is the same with the
other elements which combine with hydrogen and oxygen. Thus
sulphur gives SO3; consequently, six equivalents of oxygen fall to an
atom of sulphur, and in SH2 two equivalents of hydrogen. The sum
is again equal to eight. The relation between Cl2O7 and ClH is the
same. This shows that the property of elements of combining with
such different elements as oxygen and hydrogen is subject to one[15]
common law, which is also formulated in the system of the elements
presently to be described.[7]
In the preceding we see not only the regularity and simplicity
which govern the formation and properties of the oxides and of all the
compounds of the elements, but also a fresh and exact means for
recognising the analogy of elements. Analogous elements give compounds
of analogous types, both higher and lower. If CO2 and SO2 are
two gases which closely resemble each other both in their physical and
chemical properties, the reason of this must be looked for not in an
analogy of sulphur and carbon, but in that identity of the type
of combination, RX4, which both oxides assume, and in that influence
which a large mass of oxygen always exerts on the properties
of its compounds. In fact, there is little resemblance between carbon
and sulphur, as is seen not only from the fact that CO2 is the higher
form of oxidation, whilst SO2 is able to further oxidise into SO3, but
also from the fact that all the other compounds—for example, SH2 and
CH4, SCl2 and CCl4, &c.—are entirely unlike both in type and in
chemical properties. This absence of analogy in carbon and sulphur
is especially clearly seen in the fact that the highest saline oxides
are of different composition, CO2 for carbon, and SO3 for sulphur. In[16]
Chapter VIII. we considered the limit to which carbon tends in
its compounds, and in a similar manner there is for every element in
its compounds a tendency to attain a certain highest limit RXn. This
view was particularly developed in the middle of the present century
by Frankland in studying the metallo-organic compounds, i.e. those in
which X is wholly or partially a hydrocarbon radicle; for instance,
X = CH3 or C2H5 &c. Thus, for example, antimony, Sb (Chapter XIX.)
gives, with chlorine, compounds SbCl3 and SbCl5 and corresponding
oxygen compounds Sb2O3 and Sb2O5, whilst under the action of CH3I,
C2H5I, or in general EI (where E is a hydrocarbon radicle of the
paraffin series), upon antimony or its alloy with sodium there are
formed SbE3 (for example, Sb(CH3)3, boiling at about 81°), which,
corresponding to the lower form of combination SbX3, are able to
combine further with EI, or Cl2, or O, and to form compounds of the
limiting type SbX5; for example, SbE4Cl corresponding to NH4Cl with
the substitution of nitrogen by antimony, and of hydrogen by the
hydrocarbon radicle. The elements which are most chemically analogous
are characterised by the fact of their giving compounds of similar form
RXn. The halogens which are analogous give both higher and lower
compounds. So also do the metals of the alkalis and of the alkaline
earths. And we saw that this analogy extends to the composition and
properties of the nitrogen and hydrogen compounds of these metals,
which is best seen in the salts. Many such groups of analogous
elements have long been known. Thus there are analogues of oxygen,
nitrogen, and carbon, and we shall meet with many such groups. But
an acquaintance with them inevitably leads to the questions, what
is the cause of analogy and what is the relation of one group to
another? If these questions remain unanswered, it is easy to fall into
error in the formation of the groups, because the notions of the degree
of analogy will always be relative, and will not present any accuracy
or distinctness Thus lithium is analogous in some respects to
potassium and in others to magnesium; beryllium is analogous to
both aluminium and magnesium. Thallium, as we shall afterwards
see and as was observed on its discovery, has much kinship with
lead and mercury, but some of its properties appertain to lithium and
potassium. Naturally, where it is impossible to make measurements
one is reluctantly obliged to limit oneself to approximate comparisons,
founded on apparent signs which are not distinct and are wanting
in exactitude. But in the elements there is one accurately measurable
property, which is subject to no doubt—namely, that property which is
expressed in their atomic weights. Its magnitude indicates the relative
mass of the atom, or, if we avoid the conception of the atom, its[17]
magnitude shows the relation between the masses forming the chemical
and independent individuals or elements. And according to the teaching
of all exact data about the phenomena of nature, the mass of a substance
is that property on which all its remaining properties must be
dependent, because they are all determined by similar conditions or
by those forces which act in the weight of a substance, and this is
directly proportional to its mass. Therefore it is most natural to seek
for a dependence between the properties and analogies of the elements
on the one hand and their atomic weights on the other.
This is the fundamental idea which leads to arranging all the
elements according to their atomic weights. A periodic repetition of properties
is then immediately observed in the elements. We are already
familiar with examples of this:—
| F |
= |
19, |
Cl |
= |
35·5, |
Br |
= |
80, |
I |
= |
127, |
| Na |
= |
23, |
K |
= |
39, |
Rb |
= |
85, |
Cs |
= |
133, |
| Mg |
= |
24, |
Ca |
= |
340, |
Sr |
= |
87, |
Ba |
= |
137. |
The essence of the matter is seen in these groups. The halogens
have smaller atomic weights than the alkali metals, and the latter
than the metals of the alkaline earths. Therefore, if all the elements
be arranged in the order of their atomic weights, a periodic repetition
of properties is obtained. This is expressed by the law of periodicity,
the properties of the elements, as well as the forms and properties
of their compounds, are in periodic dependence or (expressing ourselves
algebraically) form a periodic function of the atomic weights of
the elements.[8] Table I. of the periodic system of the elements, which is[18]
placed at the very beginning of this book, is designed to illustrate
this law. It is arranged in conformity with the eight types of
oxides described in the preceding pages, and those elements which give
the oxides, R2O and consequently salts RX, form the 1st group; the
elements giving R2O2 or RO as their highest grade of oxidation belong
to the 2nd group; those giving R2O3 as their highest oxides form the
3rd group, and so on; whilst the elements of all the groups which are
nearest in their atomic weights are arranged in series from 1 to 12.
The even and uneven series of the same groups present the same forms
and limits, but differ in their properties, and therefore two contiguous
series, one even and the other uneven—for instance, the 4th and 5th—form
a period. Hence the elements of the 4th, 6th, 8th, 10th, and 12th,
or of the 3rd, 5th, 7th, 9th, and 11th, series form analogues, like the
halogens, the alkali metals, &c. The conjunction of two series, one even[19]
and one contiguous uneven series, thus forms one large period. These
periods, beginning with the alkali metals, end with the halogens. The
elements of the first two series have the lowest atomic weights, and in
consequence of this very circumstance, although they bear the general
properties of a group, they still show many peculiar and independent
properties.[9] Thus fluorine, as we know, differs in many points from
the other halogens, and lithium from the other alkali metals, and
so on. These lightest elements may be termed typical elements. They
include—
H.
Li, Be, B, C, N, O, F.
Na, Mg....
In the annexed table all the remaining elements are arranged, not
in groups and series, but according to periods. In order to understand
the essence of the matter, it must be remembered that here the
atomic weight gradually increases along a given line; for instance, in
the line commencing with K = 39 and ending with Br = 80, the intermediate
elements have intermediate atomic weights, as is clearly seen
in Table III., where the elements stand in the order of their atomic
weights.
| I. |
II. |
III. |
IV. |
V. |
VI. |
VII. |
|
I. |
II. |
III. |
IV. |
V. |
VI. |
VII. |
| Even Series. |
|
| |
|
|
Mg |
Al |
Si |
P |
S |
Cl |
| K |
Ca |
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
Ga |
Ge |
As |
Se |
Br |
| Rb |
Sr |
Y |
Zr |
Nb |
Mo |
— |
Ru |
Rh |
Pd |
Ag |
Cd |
In |
Sn |
Sb |
Te |
I |
| Cs |
Ba |
La |
Ce |
Di? |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
| — |
— |
Yb |
— |
Ta |
W |
— |
Os |
Ir |
Pt |
Au |
Hg |
Tl |
Pb |
Bi |
— |
— |
| — |
— |
— |
Th |
— |
U |
|
|
| |
Uneven Series. |
The same degree of analogy that we know to exist between potassium,
rubidium, and cæsium; or chlorine, bromine, and iodine; or calcium,
strontium, and barium, also exists between the elements of the other
vertical columns. Thus, for example, zinc, cadmium, and mercury,
which are described in the following chapter, present a very close
analogy with magnesium. For a true comprehension of the matter[10] it[20]
[21]
is very important to see that all the aspects of the distribution
of the elements according to their atomic weights essentially express
one and the same fundamental dependence—periodic properties.[11] The
following points then must be remarked in it.
[22]
1. The composition of the higher oxygen compounds is determined
by the groups: the first group gives R2O, the second R2O2 or RO,
the third R2O3, &c. There are eight types of oxides and therefore
eight groups. Two groups give a period, and the same type of oxide
is met with twice in a period. For example, in the period beginning
with potassium, oxides of the composition RO are formed by calcium
and zinc, and of the composition RO3 by molybdenum and tellurium.
The oxides of the even series, of the same type, have stronger basic
properties than the oxides of the uneven series, and the latter as a
rule are endowed with an acid character. Therefore the elements
which exclusively give bases, like the alkali metals, will be found at the
commencement of the period, whilst such purely acid elements as the
halogens will be at the end of the period. The interval will be occupied
by intermediate elements, whose character and properties we shall
afterwards describe. It must be observed that the acid character is
chiefly proper to the elements with small atomic weights in the uneven[23]
series, whilst the basic character is exhibited by the heavier elements
in the even series. Hence elements which give acids chiefly predominate
among the lightest (typical) elements, especially in the last groups;
whilst the heaviest elements, even in the last groups (for instance,
thallium, uranium) have a basic character. Thus the basic and acid
characters of the higher oxides are determined (a) by the type of oxide,
(b) by the even or uneven series, and (c) by the atomic weight.[11 bis]
The groups are indicated by Roman numerals from I. to VIII.
2. The hydrogen compounds being volatile or gaseous substances
which are prone to reaction—such as HCl, H2O, H3N, and H4C[12]—are
only formed by the elements of the uneven series and higher groups
giving oxides of the forms R2On, RO3, R2O5, and RO2.
3. If an element gives a hydrogen compound, RXm, it forms an
organo-metallic compound of the same composition, where X = CnH2n+1;
that is, X is the radicle of a saturated hydrocarbon. The elements of
the uneven series, which are incapable of giving hydrogen compounds,
and give oxides of the forms RX, RX2, RX3, also give organo-metallic
compounds of this form proper to the higher oxides. Thus[24]
zinc forms the oxide ZnO, salts ZnX2 and zinc ethyl Zn(C2H5)2. The
elements of the even series do not seem to form organo-metallic compounds
at all; at least all efforts for their preparation have as yet
been fruitless—for instance, in the case of titanium, zirconium, or
iron.
4. The atomic weights of elements belonging to contiguous periods
differ approximately by 45; for example, K<Rb, Cr<Mo, Br<I.
But the elements of the typical series show much smaller differences.
Thus the difference between the atomic weights of Li, Na, and K,
between Ca, Mg, and Be, between Si and C, between S and O, and
between Cl and F, is 16. As a rule, there is a greater difference
between the atomic weights of two elements of one group and belonging
to two neighbouring series (Ti-Si = V-P = Cr-S = Mn-Cl
= Nb-As, &c. = 20); and this difference attains a maximum with the
heaviest elements (for example, Th-Pb = 26, Bi-Ta = 26, Ba-Cd
= 25, &c.). Furthermore, the difference between the atomic weights
of the elements of even and uneven series also increases. In fact, the
differences between Na and K, Mg and Ca, Si and Ti, are less abrupt
than those between Pb and Th, Ta and Bi, Cd and Ba, &c. Thus even
in the magnitude of the differences of the atomic weights of analogous
elements there is observable a certain connection with the gradation of
their properties.[12 bis]
5. According to the periodic system every element occupies a certain
position, determined by the group (indicated in Roman numerals)
and series (Arabic numerals) in which it occurs. These indicate the
atomic weight, the analogues, properties, and type of the higher oxide,
and of the hydrogen and other compounds—in a word, all the chief
quantitative and qualitative features of an element, although there yet
remain a whole series of further details and peculiarities whose cause[25]
should perhaps be looked for in small differences of the atomic weights.
If in a certain group there occur elements, R1, R2, R3, and if in that
series which contains one of these elements, for instance R2, an
element Q2 precedes it and an element T2 succeeds it, then the properties
of R2 are determined by the properties of R1, R3, Q2, and T2.
Thus, for instance, the atomic weight of R2 = ¼(R1 + R3 + Q2 + T2).
For example, selenium occurs in the same group as sulphur, S = 32, and
tellurium, Te = 125, and, in the 7th series As = 75 stands before it and
Br = 80 after it. Hence the atomic weight of selenium should be
¼(32 + 125 + 75 + 80) = 78, which is near to the truth. Other properties
of selenium may also be determined in this manner. For example,
arsenic forms H3As, bromine gives HBr, and it is evident that selenium,
which stands between them, should form H2Se, with properties intermediate
between those of H3As and HBr. Even the physical
properties of selenium and its compounds, not to speak of their composition,
being determined by the group in which it occurs, may be foreseen
with a close approach to reality from the properties of sulphur, tellurium,
arsenic, and bromine. In this manner it is possible to foretell the properties
of still unknown elements. For instance in the position IV, 5—that
is, in the IVth group and 5th series—an element is still wanting.
These unknown elements may be named after the preceding known element
of the same group by adding to the first syllable the prefix eka-,
which means one in Sanskrit. The element IV, 5, follows after IV, 3,
and this latter position being occupied by silicon, we call the unknown
element ekasilicon and its symbol Es. The following are
the properties which this element should have on the basis of the
known properties of silicon, tin, zinc, and arsenic. Its atomic weight
is nearly 72, higher oxide EsO2, lower oxide EsO, compounds of the
general form EsX4, and chemically unstable lower compounds of the
form EsX2. Es gives volatile organo-metallic compounds—for instance,
Es(CH3)4, Es(CH3)3Cl, and Es(C2H5)4, which boil at about 160°, &c.;
also a volatile and liquid chloride, EsCl4, boiling at about 90° and of
specific gravity about 1·9. EsO2 will be the anhydride of a feeble colloidal
acid, metallic Es will be rather easily obtainable from the oxides
and from K2EsF6 by reduction, EsS2 will resemble SnS2 and SiS2, and
will probably be soluble in ammonium sulphide; the specific gravity
of Es will be about 5·5, EsO2 will have a density of about 4·7, &c.
Such a prediction of the properties of ekasilicon was made by me in
1871, on the basis of the properties of the elements analogous to it:
IV, 3, = Si, IV, 7 = Sn, and also II, 5 = Zn and V, 5 = As. And now
that this element has been discovered by C. Winkler, of Freiberg, it
has been found that its actual properties entirely correspond with those[26]
which were foretold.[13] In this we see a most important confirmation
of the truth of the periodic law. This element is now called germanium,
Ge (see Chapter XVIII.). It is not the only one that has been
predicted by the periodic law.[14] We shall see in describing the elements
of the third group that properties were foretold of an element ekaaluminium,
III, 5, El = 68, and were afterwards verified when the
metal termed ‘gallium’ was discovered by De Boisbaudran. So also the
properties of scandium corresponded with those predicted for ekaboron,
according to Nilson.[15]
[27]
6. As a true law of nature is one to which there are no exceptions,
the periodic dependence of the properties on the atomic weights
of the elements gives a new means for determining by the equivalent
the atomic weight or atomicity of imperfectly investigated but
known elements, for which no other means could as yet be applied
for determining the true atomic weight. At the time (1869) when the
periodic law was first proposed there were several such elements. It
thus became possible to learn their true atomic weights, and these were
verified by later researches. Among the elements thus concerned were
indium, uranium, cerium, yttrium, and others.
7. The periodic variability of the properties of the elements in
dependence on their masses presents a distinction from other kinds
of periodic dependence (as, for example, the sines of angles vary
periodically and successively with the growth of the angles, or the
temperature of the atmosphere with the course of time), in that the
weights of the atoms do not increase gradually, but by leaps; that is,
according to Dalton's law of multiple proportions, there not only are
not, but there cannot be, any transitive or intermediate elements between[28]
two neighbouring ones (for example, between K = 39 and Ca = 40, or
Al = 27 and Si = 28, or C = 12 and N = 14, &c.) As in a molecule
of a hydrogen compound there may be either one, as in HF, or two, as
in H2O, or three, as in NH3, &c., atoms of hydrogen; but as there cannot
be molecules containing 2½ atoms of hydrogen to one atom of another
element, so there cannot be any element intermediate between N and
O, with an atomic weight greater than 14 or less than 16, or between
K and Ca. Hence the periodic dependence of the elements cannot be
expressed by any algebraical continuous function in the same way that
it is possible, for instance, to express the variation of the temperature
during the course of a day or year.
8. The essence of the notions giving rise to the periodic law consists
in a general physico-mechanical principle which recognises the
correlation, transmutability, and equivalence of the forces of nature.
Gravitation, attraction at small distances, and many other phenomena
are in direct dependence on the mass of matter. It might therefore have
been expected that chemical forces would also depend on mass. A dependence
is in fact shown, the properties of elements and compounds
being determined by the masses of the atoms of which they are formed.
The weight of a molecule, or its mass, determines, as we have seen,
(Chapter VII. and elsewhere) many of its properties independently of
its composition. Thus carbonic oxide, CO, and nitrogen, N2, are two
gases having the same molecular weight, and many of their properties
(density, liquefaction, specific heat, &c.) are similar or nearly similar.
The differences dependent on the nature of a substance play another
part, and form magnitudes of another order. But the properties of
atoms are mainly determined by their mass or weight, and are in
dependence upon it. Only in this case there is a peculiarity in the
dependence of the properties on the mass, for this dependence is determined
by a periodic law. As the mass increases the properties
vary, at first successively and regularly, and then return to their
original magnitude and recommence a fresh period of variation like
the first. Nevertheless here as in other cases a small variation of the
mass of the atom generally leads to a small variation of properties, and
determines differences of a second order. The atomic weights of cobalt
and nickel, of rhodium, ruthenium, and palladium, and of osmium,
iridium, and platinum, are very close to each other, and their properties
are also very much alike—the differences are not very perceptible.
And if the properties of atoms are a function of their weight,
many ideas which have more or less rooted themselves in chemistry
must suffer change and be developed and worked out in the sense of
this deduction. Although at first sight it appears that the chemical[29]
elements are perfectly independent and individual, instead of this idea
of the nature of the elements, the notion of the dependence of their properties
upon their mass must now be established; that is to say, the subjection
of the individuality of the elements to a common higher principle
which evinces itself in gravity and in all physico-chemical phenomena.
Many chemical deductions then acquire a new sense and significance,
and a regularity is observed where it would otherwise escape
attention. This is more particularly apparent in the physical properties,
to the consideration of which we shall afterwards turn, and we
will now point out that Gustavson first (Chapter X., Note 28) and
subsequently Potilitzin (Chapter XI., Note 66) demonstrated the direct
dependence of the reactive power on the atomic weight and that fundamental
property which is expressed in the forms of their compounds,
whilst in a number of other cases the purely chemical relations of elements
proved to be in connection with their periodic properties. As
a case in point, it may be mentioned that Carnelley remarked a dependence
of the decomposability of the hydrates on the position of the
elements in the periodic system; whilst L. Meyer, Willgerodt, and
others established a connection between the atomic weight or the
position of the elements in the periodic system and their property of
serving as media in the transference of the halogens to the hydrocarbons.[16]
Bailey pointed out a periodicity in the stability (under the
action of heat) of the oxides, namely: (a) in the even series (for
instance, CrO3, MoO3, WO3, and UO3) the higher oxides of a given
group decompose with greater ease the smaller the atomic weight,
while in the uneven series (for example, CO2, GeO2, SnO2, and PbO2)
the contrary is the case; and (b) the stability of the higher saline
oxides in the even series (as in the fourth series from K2O to
Mn2O7) decreases in passing from the lower to the higher groups,
while in the uneven series it increases from the Ist to the IVth group,
and then falls from the IVth to the VIIth; for instance, in the series[30]
Ag2O, CdO, In2O3, SnO2, and then SnO2, Sb2O5, TeO3, I2O7.
K. Winkler looked for and actually found (1890) a dependence between
the reducibility of the metals by magnesium and their position in the
periodic system of the elements. The greater the attention paid to
this field the more often is a distinct connection found between the
variation of purely chemical properties of analogous substances and
the variation of the atomic weights of the constituent elements and
their position in the periodic system. Besides, since the periodic
system has become more firmly established, many facts have been
gathered, showing that there are many similarities between Sn and
Pb, B and Al, Cd and Hg, &c., which had not been previously observed,
although foreseen in some cases, and a consequence of the periodic law.
Keeping our attention in the same direction, we see that the most
widely distributed elements in nature are those with small atomic
weights, whilst in organisms the lightest elements exclusively predominate
(hydrogen, carbon, nitrogen, oxygen), whose small mass facilitates
those transformations which are proper to organisms. Poluta
(of Kharkoff), C. C. Botkin, Blake, Brenton, and others even discovered
a correlation between the physiological action of salts and other reagents
on organisms and the positions occupied in the periodic system
by the metals contained in them.[17]
As, from the necessity of the case, the physical properties must be
in dependence on the composition of a substance, i.e. on the quality
and quantity of the elements forming it, so for them also a dependence
on the atomic weight of the component elements must be
expected, and consequently also on their periodic distribution. We
shall meet with repeated proofs of this in the further exposition of
our treatise, and for the present will content ourselves with citing
the discovery by Carnelley in 1879 of the dependence of the magnetic
properties of the elements on the position occupied by them in the
periodic system. Carnelley showed that all the elements of the even[31]
series (beginning with lithium, potassium, rubidium, cæsium) belong
to the number of magnetic (paramagnetic) substances; for example,
according to Faraday and others,[17 bis] C, N, O, K, Ti, Cr, Mn, Fe, Co,
Ni, Ce, are magnetic; and the elements of the uneven series are
diamagnetic, H, Na, Si, P, S, Cl, Cu, Zn, As, Se, Br, Ag, Cd, Sn, Sb,
I, Au, Hg, Tl, Pb, Bi.
Carnelley also showed that the melting-point of elements varies
periodically, as is seen by the figures in Table III. (nineteenth column),[18]
where all the most trustworthy data are collected, and predominance
is given to those having maximum and minimum values.[19]
[32]
[33]
There is no doubt that many other physical properties will, when
further studied, also prove to be in periodic dependence on the atomic
weights,[19 bis] but at present only a few are known with any completeness,
and we will only refer to the one which is the most easily and
frequently determined—namely, the specific gravity in a solid and
liquid state, the more especially as its connection with the chemical
properties and relations of substances is shown at every step. Thus,
for instance, of all the metals those of the alkalis, and of all the non-metals
the halogens, are the most energetic in their reactions, and they
have the lowest specific gravity among the adjacent elements, as is seen
in Table III., column 17. Such are sodium, potassium, rubidium,
cæsium among the metals, and chlorine, bromine, and iodine among the
non-metals; and as such less energetic metals as iridium, platinum,
and gold (and even charcoal or the diamond) have the highest specific
gravity among the elements near to them in atomic weight; therefore
the degree of the condensation of matter evidently influences the
course of the transformations proper to a substance, and furthermore
this dependence on the atomic weight, although very complex, is of a
clearly periodic character. In order to account for this to some extent,
it may be imagined that the lightest elements are porous, and, like a
sponge, are easily penetrated by other substances, whilst the heavier
elements are more compressed, and give way with difficulty to the
insertion of other elements. These relations are best understood when,
instead of the specific gravities referring to a unit of volume,[20] the
atomic volumes of the elements—that is, the quotient A/d of the atomic[34]
weight A by the specific gravity d—are taken for comparison. As,
according to the entire sense of the atomic theory, the actual matter
of a substance does not fill up its whole cubical contents, but is surrounded
by a medium (ethereal, as is generally imagined), like the stars
and planets which travel in the space of the heavens and fill it, with
greater or less intervals, so the quotient A/d only expresses the mean
volume corresponding to the sphere of the atoms, and therefore [3root]A/d
is the mean distance between the centres of the atoms. For compounds
whose molecules weigh M, the mean magnitude of the atomic volume is
obtained by dividing the mean molecular volume M/d by the number
of atoms n in the molecule.[21] The above relations may easily be
expressed from this point of view by comparing the atomic volumes.
Those comparatively light elements which easily and frequently enter
into reaction have the greatest atomic volumes: sodium 23, potassium
45, rubidium 57, cæsium 71, and the halogens about 27; whilst with
those elements which enter into reaction with difficulty, the mean atomic
volume is small; for carbon in the form of a diamond it is less than
4, as charcoal about 6, for nickel and cobalt less than 7, for iridium
and platinum about 9. The remaining elements having atomic weights
and properties intermediate between those elements mentioned above
have also intermediate atomic volumes. Therefore the specific gravities
and specific volumes of solids and liquids stand in periodic dependence
on the atomic weights, as is seen in Table III., where both A (the
atomic weight) and d (the specific gravity), and A/d (specific volumes
of the atoms) are given (column 18).
Thus we find that in the large periods beginning with lithium,
sodium, potassium, rubidium, cæsium, and ending with fluorine, chlorine,
bromine, iodine, the extreme members (energetic elements) have a
small density and large volume, whilst the intermediate substances
gradually increase in density and decrease in volume—that is, as the
atomic weight increases the density rises and falls, again rises and falls,[35]
and so on. Furthermore, the energy decreases as the density rises, and
the greatest density is proper to the atomically heaviest and least
energetic elements; for example, Os, Ir, Pt, Au, U.
In order to explain the relation between the volumes of the elements
and of their compounds, the densities (column S) and volumes
(column M/s) of some of the higher saline oxides arranged in the same
order as in the case of the elements are given on p. 36. For convenience
of comparison the volumes of the oxides are all calculated
per two atoms of an element combined with oxygen. For example,
the density of Al2O3 = 4·0, weight Al2O3 = 102, volume Al2O3 = 25·5.
Whence, knowing the volume of aluminium to be 11, it is at once seen
that in the formation of aluminium oxide, 22 volumes of it give 25·5
volumes of oxide. A distinct periodicity may also be observed with
respect to the specific gravities and volumes of the higher saline oxides.
Thus in each period, beginning with the alkali metals, the specific
gravity of the oxides first rises, reaches a maximum, and then falls on
passing to the acid oxides, and again becomes a minimum about the
halogens. But it is especially important to call attention to the fact
that the volume of the alkali oxides is less than that of the metal contained
in them, which is also expressed in the last column, giving this
difference for each atom of oxygen.[22] Thus 2 atoms of sodium, or
46 volumes, give 24 volumes of Na2O, and about 37 volumes of 2NaHO—that
is, the oxygen and hydrogen in distributing themselves in the
medium of sodium have not only not increased the distance between
its atoms, but have brought them nearer together, have drawn them
together by the force of their great affinity, by reason, it may be
presumed, of the small mutual attraction of the atoms of sodium.
Such metals as aluminium and zinc, in combining with oxygen and
forming oxides of feeble salt-forming capacity, hardly vary in volume,
but the common metals and non-metals, and especially those forming
acid oxides, always give an increased volume when oxidised—that is,
the atoms are set further apart in order to make room for the oxygen.
The oxygen in them does not compress the molecule as in the alkalis;
it is therefore comparatively easily disengaged.
[36]
| |
s |
M/s |
Volume of Oxygen |
| H2O |
1·0 |
18 |
?-22 |
| Li2O |
2·0 |
15 |
-9 |
| Be2O2 |
3·06 |
16 |
+2·6 |
| B2O3 |
1·8 |
39 |
+10·0 |
| C2O4 |
1·6 |
55 |
+10·6 |
| N2O5 |
1·64 |
66 |
?+4 |
| |
| Na2O |
2·6 |
24 |
-22 |
| Mg2O2 |
3·5 |
23 |
-4·5 |
| Al2O3 |
4·0 |
26 |
+1·3 |
| Si2O4 |
2·65 |
45 |
+5·2 |
| P2O5 |
2·39 |
59 |
+6·2 |
| S2O6 |
1·96 |
82 |
+8·7 |
| Cl2O7 |
?1·92 |
95 |
+6 |
| |
| K2O |
2·7 |
35 |
-36 |
| Ca2O2 |
3·25 |
34 |
-8 |
| Sc2O3 |
3·86 |
35 |
?0 |
| Ti2O4 |
4·2 |
38 |
+3 |
| V2O5 |
3·49 |
52 |
+6·7 |
| Cr2O6 |
2·74 |
73 |
+9·5 |
| Cu2O |
5·9 |
24 |
+9·6 |
| Zn2O2 |
5·7 |
23 |
+4·8 |
| Ga2O3 |
?5·1 |
36 |
+4 |
| Ge2O4 |
4·7 |
44 |
+4·5 |
| As2O5 |
4·1 |
56 |
+6·0 |
| |
| Sr2O2 |
4·7 |
44 |
-13 |
| Y2O3 |
5·0 |
45 |
?-2 |
| Zr2O4 |
5·5 |
44 |
0 |
| Nb2O5 |
4·7 |
57 |
+6 |
| MoO6 |
4·4 |
65 |
+6·8 |
| Ag2O |
7·5 |
31 |
+11 |
| Cd2O2 |
8·0 |
32 |
+3 |
| In2O3 |
7·18 |
38 |
+2·7 |
| Sn2O4 |
7·0 |
43 |
+2·7 |
| Sb2O5 |
6·5 |
49 |
+2·6 |
| TeO6 |
5·1 |
68 |
+4·7 |
| |
| Ba2O2 |
5·7 |
52 |
-10 |
| La2O3 |
6·5 |
50 |
+1 |
| Ce2O4 |
6·74 |
50 |
+2 |
| Ta2O5 |
7·5 |
59 |
+4·6 |
| W2O6 |
6·8 |
68 |
+8·2 |
| Hg2O2 |
11·1 |
39 |
+4·5 |
| Pb2O4 |
8·9 |
53 |
+4·2 |
| Th2O4 |
9·86 |
54 |
+2 |
[37]
As the volumes of the chlorides, organo-metallic and all other
corresponding compounds, also vary in a like periodic succession with a
change of elements, it is evidently possible to indicate the properties
of substances yet uninvestigated by experimental means, and even those
of yet undiscovered elements. It was possible by following this method
to foretell, on the basis of the periodic law, many of the properties of
scandium, gallium, and germanium, which were verified with great
accuracy after these metals had been discovered.[23] The periodic law,
therefore, has not only embraced the mutual relations of the elements
and expressed their analogy, but has also to a certain extent subjected
to law the doctrine of the types of the compounds formed by the
elements: it has enabled us to see a regularity in the variation of
all chemical and physical properties of elements and compounds, and
has rendered it possible to foretell the properties of elements and
compounds yet uninvestigated by experimental means; thus it has
prepared the ground for the building up of atomic and molecular
mechanics.[24]
[39]
CHAPTER XVI
ZINC, CADMIUM, AND MERCURY
These three metals give, like magnesium, oxides RO, which form
feebly energetic bases, and like magnesium they are volatile. The
volatility increases with the atomic weight. Magnesium can be distilled
at a white heat, zinc at a temperature of about 930°, cadmium
about 770°, and mercury about 351°. Their oxides, RO, are more
easily reducible than magnesia, and mercuric oxide is the most easily
reducible. The properties of their salts RX2 are very similar to
the properties of MgX2. Their solubility, power of forming double
and basic salts, and many other qualities are in many respects identical
with those of MgX2. The greater or less ease with which they are
oxidised, the instability of their compounds, the density of the metals
and their compounds, their scarcity in nature, and many other properties
gradually change with the increase of atomic weight, as might be
expected from the periodicity of the elements. Their principal characteristics,
as contrasted with magnesium, find a general expression in the
fact that zinc, cadmium, and mercury are heavy metals.
Zinc stands nearest to magnesium in atomic weight and in properties.
Thus zinc sulphate, or white vitriol, easily crystallises with
seven molecules of water, ZnSO4,7H2O. It is isomorphous with Epsom
salts, and parts with difficulty with the last molecule of water; it
forms double salts—for instance, ZnK2(SO4)2,6H2O—exactly as magnesium
sulphate does.[1] Zinc oxide, ZnO, is a white powder, almost insoluble[40]
in water,[2] like magnesia, from which, however, it is distinguished
by its solubility in solutions of sodium and potassium hydroxides.[3]
Zinc chloride[4] is decomposed by water, combines with ammonium[41]
chloride, potassium chloride, &c., just like magnesium chloride, forms
an oxychloride, and also combines with zinc oxide.[4 bis]
Zinc, like many heavy metals, is often found in nature in combination[42]
with sulphur, forming the so-called zinc blende,[5] ZnS. It sometimes
occurs in large masses, often crystallised in cubes; it is frequently
translucent, and has a metallic lustre, although this is not so clearly
developed as in many other metallic sulphides with which we shall hereafter
become acquainted. The ores of zinc also comprise the carbonate,
calamine, and silicate, siliceous calamine.
Fig. 80.—Distillation of zinc in a
crucible placed in a furnace. o c, tube along which the vapour passes and condenses.
Metallic zinc (spelter) is most frequently obtained from the ores
containing the carbonate[6]—that is, from calamine, which is sometimes
found in thick veins: for instance, in
Poland, Galicia, in some places on the
banks of the Rhine, and in considerable
masses in Belgium and England. In
Russia beds of zinc ore are met with in
Poland and the Caucasus, but the output
is small. In Sweden, as early as the
fifteenth century, calamine was worked
up into an alloy of zinc and copper (brass),
and Paracelsus produced zinc from calamine;
but the technical production of the
metal itself, long ago practised in China,
only commenced in Europe in 1807—in
Belgium, when the Abbé Donnet discovered
that zinc was volatile. From that time
the production increased until it is now about 150 million kilograms in
Germany alone.
The reduction of metallic zinc from its ores is based on the fact that
zinc oxide[7] is easily reduced by charcoal at a red heat: ZnO + C[43]
= Zn + CO. The zinc thus obtained is in a finely divided state and
impure, being mixed with other metals reduced with it, but the greater
portion is converted into vapour, from which it easily passes into a
liquid or solid state. The reduction and distillation are carried on in
earthenware retorts, filled with a mixture of the divided ore and
charcoal. The vapours of zinc and gases formed during the reaction
escape by means of a pipe leading downwards, and are led to a chamber
where the vapours are cooled. By this means they do not come into
contact with the air, because the neck of the retort is filled with
gaseous carbonic oxide, and therefore the zinc does not oxidise; otherwise
its vapour would burn in the air.[7 bis] The vapours of zinc, entering
into the cooling chamber, condense into white zinc powder or zinc
dust. When the neck of the retort is heated the zinc is obtained in
a liquid state, and is cast into plates, in which form it is generally
sold.
Commercial zinc is generally impure, containing a mixture of lead,
particles of carbon, iron, and other metals carried over with the vapours,
although they are not volatile at a temperature approaching 1000°. If
it be required to obtain pure zinc from the commercial article, it is subjected
to a further distillation in a crucible with a pipe passing through
the bottom, the vapours formed by the heated zinc only having exit
through the pipe cemented into the bottom of the crucible. Passing
through this pipe, the vapours condense to a liquid, which is collected
in a receiver. Zinc thus purified is generally re-melted and cast into
rods, and in this form is often used for physical and chemical researches
where a pure article is required.[8]
[44]
Metallic zinc has a bluish-white colour; its lustre, compared with
many other metals, is insignificant. When cast it exhibits a crystalline
structure. Its specific gravity is about 7—that is, varies from 6·8
to 7·2, according to the degree of compression (by forging, rolling, &c.)
to which it has been subjected. It is very ductile, considering its
hardness. For this reason it chokes up files when being worked. Its
malleability is considerable when pure, but in the ordinary impure condition
in which it is sold, it is impossible to roll it at the ordinary
temperature, as it easily breaks. At a temperature of 100°, however,
it easily undergoes such operations, and can then be drawn into wire
or rolled into sheets. If heated further it again becomes brittle, and
at 200° may be even crushed into powder, so completely does it lose
its molecular cohesion. It melts at 418°, and distils at 930°.
Zinc does not undergo any change in the atmosphere. Even in
very damp air it only becomes slowly coated with a very thin white
coating of oxide. For this reason it is available for all objects which
are only in contact with air. Therefore sheet zinc may be used for
roofing and many other purposes.[9] This great unchangeability of zinc
in the air shows its slight energy with regard to oxygen compared
with the metals already mentioned, which are capable of reducing zinc
from solutions. But zinc plays this part with regard to the remaining
metals—for example, it reduces salts of lead, copper, mercury, &c.
Although zinc is an almost unoxidisable metal at the ordinary
temperature, it burns in the air on being heated, particularly when
in the form of shavings or in the condition of vapour. At the
ordinary temperature zinc does not decompose water—at any rate, if
the metal be in a dense mass. But even at a temperature of 100° zinc
begins little by little to decompose water; it easily displaces the
hydrogen of acids at the ordinary temperature, and of alkalis on
being heated.
In this respect the action of zinc varies a great deal with the
degree of its purity. Weak sulphuric acid (corresponding with the
composition H2SO4,8H2O) at the ordinary temperature does not act at
all on chemically pure zinc, and even a stronger solution acts very
slowly. If the temperature be raised, and particularly if the zinc be
previously slightly heated, so as to cover the surface with a film of
oxide, chemically pure zinc acts on sulphuric acid. Thus, for example,
one cubic centimetre of zinc in sulphuric acid having a composition[45]
H2SO4,6H2O at the ordinary temperature in two hours only dissolves
to the extent of 0·018 gram, and at a temperature of 100° about
3·5 grams. If we compare this slow action with that rapid evolution
of hydrogen which occurs in the case of commercial zinc, we see that
the influence of those impurities in the zinc is very great. Every
particle of charcoal or iron introduced into the mass of the zinc, and
likewise the connection of the zinc with a piece of another electro-negative
metal, assists such a dissolution. The slowness of the action
of sulphuric acid on pure zinc (and likewise on amalgamated zinc) may
also be explained by the fact that a layer of hydrogen[10] collects on
the surface of the metal, preventing contact between the acid and the
metal.[10 bis]
The action of zinc on acids, and the consequent formation of zinc[46]
salts, interferes with its application in many cases, particularly for the
preservation of liquids either containing or capable of developing
acid. For this reason zinc vessels ought not to be used for the preparation
or preservation of food, as this often contains acids which form[47]
poisonous salts with the zinc. Even ordinary water, containing carbonic
acid, slowly attacks zinc.
Finely divided zinc, or zinc dust, obtained in the distillation of
the metal when the receiver is not heated up to the melting point, on
account of its presenting a large surface of contact and containing
foreign matter (particularly zinc oxide), has in the highest degree the
property of decomposing acids, and even water, which it easily decomposes,
particularly if slightly heated. On this account zinc dust is
often used in laboratories and factories as a reducing agent. A similar
influence of the finely divided state is also noticed in other metals—for
instance, copper and silver—which again shows the close connection
between chemical and physico-mechanical phenomena. We
must first of all turn to this close connection for an explanation
of the widely spread application of zinc in galvanic batteries, where
the chemical (latent, potential) energy of the acting substances is transformed
into (evident, kinetic) galvanic energy, and through this latter
into heat, light, or mechanical work.
Hermann and Stromeyer, in 1819, showed that cadmium is almost
always found with zinc, and in many respects resembles it. When
distilled the cadmium volatilises sooner, because it has a lower boiling
point. Sometimes the zinc dust obtained by the first distillation of
zinc contains as much as 5 per cent. of cadmium. When zinc blende,
containing cadmium, is roasted, the zinc passes into the state of
oxide, and the cadmium sulphide in the ore oxidises into cadmium
sulphate, CdSO4, which resists tolerably well the action of heat; therefore
if roasted zinc blende be washed with water, a solution of
cadmium sulphate will be obtained, from which it is very easy to
prepare metallic cadmium. Hydrogen sulphide may be used for
separating cadmium from its solutions; it gives a yellow precipitate
of cadmium sulphide, CdS (according to the equation CdSO4 + H2S
= H2SO4 + CdS),[11] which, on account of its characteristic colour, is
used as a pigment.[11 bis] Cadmium sulphide, when strongly heated in
air, leaves cadmium oxide, from which the metal may be obtained in
precisely the same way as in the case of zinc.
[48]
Cadmium is a white metal, and when freshly cut is almost as white
and lustrous as tin. It is so soft that it may be easily cut with a knife,
and so malleable that it can be easily drawn into wire, rolled into
sheets, &c. Its specific gravity is 8·67, melting point 320°, boiling
point 770°; its vapours burn, forming a brown powder of the oxide.[12]
Next to mercury it is the most volatile metal; hence Deville determined
the density of its vapours compared with hydrogen, and found
it to be equal to 57·1; therefore the molecule contains one atom whose
weight = 112. V. Meyer found the like for zinc; the molecule of
mercury also contains one atom.
Mercury resembles zinc and cadmium in many respects, but presents
that distinction from them which is always noticed in all the heaviest
metals (with regard to atomic weight and density) compared with the
lighter ones—namely, that it oxidises with more difficulty, and its
compounds are more easily decomposed.[12 bis] Besides compounds of the[49]
usual type RX2, it also gives those of the lower type, RX, which are
unknown for zinc and cadmium.[13] Mercury therefore gives salts of
the composition HgX (mercurous salts) and HgX2 (mercuric salts), the
oxides having the formulæ Hg2O and HgO respectively.
Mercury is found in nature almost exclusively in combination with
sulphur (like zinc and cadmium, but is still rarer than them) in the
form known as cinnabar, HgS (Chapter XX., Note 29). It is far more
rarely met with in the native or metallic condition, and this in all
probability has been derived from cinnabar. Mercury ore is found only
in a few places—namely, in Spain (in Almaden), in Idria, Japan, Peru,
and California. About the year 1880 Minenkoff discovered a rich bed
of cinnabar in the Bahmout district (near the station of Nikitovka),
in the Government of Ekaterinoslav, so that now Russia even exports
mercury to other countries. Cinnabar is now being worked
in Daghestan in the Caucasus. Mercury ores are easily reduced to
metallic mercury, because the combination between the metal and the
sulphur is one of but little stability. Oxygen, iron, lime, and many
other substances, when heated, easily destroy the combination. If iron
is heated with cinnabar, iron sulphide is formed; if cinnabar is heated
with lime, mercury and calcium sulphide and sulphate are formed,
4HgS + 4CaO = 4Hg + 3CaS + CaSO4. On being heated in the air, or
roasted, the sulphur burns, oxidises, forming sulphurous anhydride,
and vapours of metallic mercury are formed. Mercury is more easily
distilled than all other metals, its boiling point being about 351°, and
therefore its separation from natural admixtures, decomposed by one of
the above-mentioned methods, is effected at the expense of a comparatively
small amount of heat. The mixture of mercury vapour,
air, and products of combustion obtained is cooled in tubes (by water
or air), and the mercury condenses as liquid metal.[14]
[50]
Mercury, as everybody knows, is a liquid metal at the ordinary
temperature. In its lustre and whiteness it resembles silver.[15] At
-39° mercury is transformed into a malleable crystalline metal; at 0°
its specific gravity is 13·596, and in the solid state at -40° it is
14·39.[16] Mercury does not change in the air—that is to say, it does
not oxidise at the ordinary temperature—but at a temperature
approaching the boiling-point, as was stated in the Introduction, it
oxidises, forming mercuric oxide. Both metallic mercury and its
compounds in general produce salivation, trembling of the hands,
and other unhealthy symptoms which are found in the workmen
exposed to the influence of mercurial vapours or the dust of its compounds.
As many of the compounds of mercury decompose on being heated—for
instance, the oxide or carbonate[17]—and as zinc, cadmium, copper,
iron, and other metals separate mercury from its salts,[18] it is evident[51]
that mercury has less chemical energy than the metals already
described, even than zinc and cadmium. Nitric acid, when acting on
an excess of mercury at the ordinary temperature, gives mercurous
nitrate, HgNO3.[19] The same acid, under the influence of heat and
when in excess (nitric oxide being liberated), forms mercuric
nitrate, Hg(NO3)2. This,[20] both in its composition and properties,
resembles the salts of zinc and cadmium. Dilute sulphuric acid
does not act on mercury, but strong sulphuric acid dissolves it, with
evolution of sulphurous anhydride (not hydrogen), and on being slightly
heated with an excess of mercury it forms the sparingly soluble mercurous
sulphate, Hg2SO4; but if mercury be strongly heated with an
excess of the acid, the mercuric salt, HgSO4,[21] is formed. Alkalis do
not act on mercury, but the non-metals chlorine, bromine, sulphur, and
phosphorus easily combine with it. They form, like the acids, two series
of compounds, HgX and HgX2. The oxygen compound of the first series
is the suboxide of mercury, or mercurous oxide, Hg2O, and of the
second order the oxide HgO, mercuric oxide. The chlorine compound
corresponding with the suboxide is HgCl (calomel), and with the oxide
HgCl2 (corrosive sublimate or mercuric chloride). In the compounds
HgX, mercury resembles the metals of the first group, and more
especially silver. In the mercuric compounds there is an evident[52]
resemblance to those of magnesium, cadmium, &c. Here the atom
of mercury is bivalent, as in the type RX2.[22] Every soluble mercurous
compound (corresponding with the type of the suboxide of
mercury), HgX, forms a white precipitate of calomel, HgCl, with
hydrochloric acid or a metallic chloride, because HgCl is very slightly
soluble in water, HgX + MCl = HgCl + MX. In soluble mercuric
compounds, HgX2, hydrochloric acid and metallic chlorides do not
form a precipitate, because corrosive sublimate, HgCl2, is soluble in
water. Alkali hydroxides precipitate the yellow mercuric oxide from
a solution of HgX2, and the black mercurous oxide from HgX.
Potassium iodide forms a dirty greenish precipitate, HgI, with
mercurous salts, HgX, and a red precipitate, HgI2, with the mercuric
salts, HgX2. These reactions distinguish the mercuric
from the mercurous salts, which latter represent the transition from[53]
the mercuric salts to mercury itself, 2HgX = Hg + HgX2. The
salts, HgX, as well as HgX2, are reduced by nascent hydrogen (e.g.
from Zn + H2SO4), by such metals as zinc and copper, and also
by many reducing agents—for example, hypophosphorous acid, the
lowest grade of oxidation of phosphorus, by sulphurous anhydride,
stannous chloride, &c. Under the action of these reagents the
mercuric salts are first transformed into the mercurous salts, and
the latter are then reduced to metallic mercury. This reaction is so
delicate that it serves to detect the smallest quantity of mercury;
for instance, in cases of poisoning, the mercury is detected by immersing
a copper plate in the solution to be tested, the mercury
being then deposited upon it (more readily on passing a galvanic
current). The copper plate, on being rubbed, shows a silvery
white colour; on being heated, it yields vapours of mercury, and
then again assumes its original red colour (if it does not oxidise).
The mercurous compounds, HgX, under the action of oxidising
agents, even air, pass into mercuric compounds, especially in the
presence of acids (otherwise a basic salt is produced), 2HgX + 2HX + O
= 2HgX2 + H2O; but the mercuric compounds, when in contact with
mercury, change more or less readily, and turn into mercurous compounds,
HgX2 + Hg = 2HgX. For this reason, in order to preserve
solutions of mercurous salts, a little mercury is generally added to
them.
The lowest oxygen compound of mercury—that is, mercurous oxide,
Hg2O—does not seem to exist, for the substance precipitated in the
form of a black mass by the action of alkalis on a solution of mercurous
salts gradually separates on keeping into the yellow mercuric
oxide and metallic mercury, as does also a simple mechanical mixture
of oxide, HgO, with mercury (Guibourt, Barfoed). The other
compound of mercury with oxygen is already known to us as mercuric
oxide, HgO, obtained in the form of a red crystalline substance by the
oxidation of mercury in the air, and precipitated as a yellow powder
by the action of sodium hydroxide on solutions of salts of the type
HgX2. In this case it is amorphous and more amenable to the
action of various reagents (Chap. XI., Note 32) than when it is in the
crystalline state. Indeed, on trituration, the red oxide is changed into
a powder of a yellow colour. It is very sparingly soluble in water, and
forms an alkaline solution which precipitates magnesia from the solution
of its salts.
Mercury combines directly with chlorine, and the first product of
combination is calomel or mercurous chloride, Hg2Cl2. This is obtained,
as above stated, in the form of a white precipitate by mixing solutions[54]
of mercurous salts with hydrochloric acid or with metallic
chlorides. A precipitate of calomel is also obtained by reducing a
boiling aqueous solution of corrosive sublimate, HgCl2, with sulphurous
anhydride. It is likewise produced by heating corrosive sublimate
with mercury.[22 bis] Calomel may be distilled (although in so doing
it decomposes and recombines on cooling from a state of vapour); its
vapour density equals 118 compared with hydrogen (= 1) (see Note 23).
In the solid state its specific gravity is 7·0; it crystallises in rhombic
prisms, is colourless, but has a yellowish tint, turns brown from the
action of light, and when boiled with hydrochloric acid decomposes into
mercury and corrosive sublimate. It is used as a strong purgative.
Corrosive sublimate or mercuric chloride, HgCl2, can be obtained from
or converted into calomel by many methods.[23] An excess of chlorine
(for instance, aqua regia) converts calomel and also mercury into corrosive
sublimate. It owes its name corrosive sublimate to its volatility,
and, in medicine up to the present day, it is termed Mercurius
sublimatus seu corrosivus. The vapour density, compared with hydrogen
(= 1) is 135; therefore its molecule contains HgCl2. It forms
colourless prismatic crystals of the rhombic system, boils at 307°,
and is soluble in alcohol. It is usually prepared by subliming a
mixture of mercuric sulphate with common salt, HgSO4 + 2NaCl
= Na2SO4 + HgCl2. Corrosive sublimate combines with mercuric
oxide, forming an oxychloride or basic salt,[23 bis] of the composition[55]
HgCl2,2HgO (magnesium and zinc form similar compounds). This
compound is obtained by mixing a solution of corrosive sublimate
with mercuric oxide or with a solution of sodium bicarbonate. In
general, with both mercurous and mercuric salts, there is a marked
tendency to form basic salts.[24]
Mercury has a remarkable power of forming very unstable compounds
with ammonia, in which the mercury replaces the hydrogen,
and, if a mercuric compound be taken, its atom occupies the place of
two atoms of the hydrogen in the ammonia. Thus Plantamour and
Hirtzel showed that precipitated mercuric oxide dried at a gentle
heat, when continuously heated (up to 100°-150°) in a stream of[56]
dry ammonia, leaves a brown powder of mercuric nitride, N2Hg3,
according to the equation 3HgO + 2NH3 = N2Hg3 + 3H2O.[24 bis] This
substance, which is attacked by water, acids, and alkalis (giving a
white powder), is very explosive when struck or rubbed, evolving
nitrogen, proving that the bond between the mercury and the nitrogen
is very feeble.[25] By the action of liquefied ammonia on yellow mercuric[57]
oxide Weitz also obtained an explosive compound, dimercurammonium
hydroxide, N2Hg4O, which corresponds with an ammonium oxide,
(NH4)2O, in which the whole of the hydrogen is replaced by mercury.
A solution of ammonia reacts with mercuric oxide, forming the
hydroxide, NHg2.OH, to which a whole series of salts, NHg2X, correspond;
these are generally insoluble in water and capable of decomposing
with an explosion. But salts of the same type, but with one atom
of mercury, NH2HgX, are more frequently and more easily formed;
they were principally studied by Kane, although known much earlier.
Thus, if ammonia be added to a solution of corrosive sublimate
(or, still better, in reverse order), a precipitate is obtained known as
white precipitate (Mercurius præcipitatus albus) or mercurammonium
chloride, NH2HgCl, which may also be regarded not only as sal-ammoniac
with the substitution of H2 by mercury, but also as HgX2, where
one X represents Cl and the other X represents the ammonia radicle,
HgCl2 + 2NH3 = NH2.HgCl + NH4Cl. When heated, mercurammonium
chloride decomposes, yielding mercurous chloride; when
heated with dry hydrochloric acid it forms ammonium chloride and
mercuric chloride. Other simple and double salts of mercurammonium,
NH2HgX, are also known. Pici (1890) showed that all the compounds
HgH2NX may be regarded as compounds of the above-named Hg2NX
with NH4X because their sum equals 2HgH2X.[25 bis]
[58]
Mercury as a liquid metal is capable of dissolving other metals and
forming metallic solutions. These are generally called ‘amalgams.’ The
formation of these solutions is often accompanied by the development
of a large amount of heat—for instance, when potassium and sodium
are dissolved (Chapter XII., Note 39); but sometimes heat is absorbed,
as, for instance, when lead is dissolved. It is evident that phenomena
of this kind are exceedingly similar to the phenomena accompanying
the dissolution of salts and other substances in water, but here it is
easy to demonstrate that which is far more difficult to observe in the
case of salts: the solution of metals in mercury is accompanied by the
formation of definite chemical compounds of the mercury with the
metals dissolved. This is shown by the fact that when pressed (best
of all in chamois leather) such solutions leave solid, definite compounds
of mercury with metals. It is, however, very difficult to obtain
them in a pure state, on account of the difficulty of separating the last
traces of mercury, which is mechanically distributed between the
crystals of the compounds. Nevertheless, in many cases such compounds
have undoubtedly been obtained, and their existence is
clearly shown by the evident crystalline structure and characteristic
appearance of many amalgams. Thus, for instance, if about 2½ p.c. of
sodium be dissolved in mercury, a hard, crystalline amalgam is obtained,
very friable and little changeable in air. It contains the compound
NaHg5 (Chapter XII., Note 39). Water decomposes it, with the evolution
of hydrogen, but more slowly than other sodium amalgams, and
this action of water only shows that the bond between the sodium
and the mercury is weak, just like the connection between mercury
and many other elements—for instance, nitrogen. Mercury directly
and easily dissolves potassium, sodium, zinc, cadmium, tin, gold, bismuth,
lead, &c., and from such solutions or alloys it is in most cases
easy to extract definite compounds—thus, for instance, the compounds
of mercury and silver have the compositions HgAg and Ag2Hg3.
Objects made of copper when rubbed with mercury become covered
with a white coating of that metal, which slowly forms an amalgam;
silver acts in the same way, but more slowly, and platinum combines
with mercury with still greater difficulty. This metal only readily
forms an amalgam when in the form of a fine powder. If salts of
platinum in solution are poured on to an amalgam of sodium, the
latter element reduces the platinum, and the platinum separated is
dissolved by the mercury. Almost all metals readily form amalgams
if their solutions are decomposed by a galvanic current, where mercury
forms the negative pole. In this way an amalgam may even
be made with iron, although iron in a mass does not dissolve in[59]
mercury. Some amalgams are found in nature—for instance, silver
amalgams. Amalgams are used in considerable quantities in the arts.
Thus the solubility of silver in mercury is taken advantage of for
extracting that metal from the ore by means of amalgamation, and
for silvering by fire. The same is the case with gold. Tin amalgam,
which is incapable of crystallising and is obtained by dissolving
tin in mercury, composes the brilliant coating of ordinary looking-glasses,
which is made to adhere to the surface of the polished
glass by simply pressing by mechanical means sheets of tin foil bathed
in mercury on to the cleansed surface of the glass.[26] (See ‘The
Nature of Amalgams,’ by W. L. Dudley; Toronto, 1889.)
[60]
CHAPTER XVII
BORON, ALUMINIUM, AND THE ANALOGOUS METALS OF THE THIRD GROUP
If the elements of small atomic weight which we have hitherto
discussed be placed in order, it will be clearly seen that, judging
by the formulæ of their higher compounds, one element is wanting
between beryllium and carbon. For lithium gives LiX, beryllium
forms BeX2, and then comes carbon giving CX4. Evidently to
complete the series we must look for an element forming RX3, and
having an atomic weight greater than 9 and less than 12. And boron
is such a one; its atomic weight is 11, and its compounds are expressed
by BX3. Lithium and beryllium are metals; carbon has no metallic
properties; boron appears in a free state in several forms which are
intermediate between the metals and non-metals. Lithium gives an
energetic caustic oxide, beryllium forms a very feeble base; hence one
would expect to find that the oxide of boron, B2O3, has still more
feeble basic properties and some acid properties, all the more as CO2
and N2O5, which follow after B2O3 in their composition and in the
periodic system, are acid oxides. And, indeed, the only known oxide of
boron exhibits a feeble basic character, together with the properties of
a feeble acid oxide. This is even seen from the fact that a solution of
boron oxide reddens blue litmus and acts on turmeric paper as an
alkali, and these reactions may be used for determining the presence of
B2O3 in solutions. By themselves the alkali borates have an alkaline
reaction, which clearly indicates the feeble acid character of boric acid.
If they are mixed in solution with hydrochloric acid, boric acid is
liberated, and if a piece of turmeric paper be immersed in this solution
and then dried, the excess of hydrochloric acid volatilises, while the
boric acid remains on the paper and communicates a brown coloration to
it, just like alkalis.
Boron trioxide or boric anhydride enters into the composition of
many minerals, in the majority of cases in small quantities as an
isomorphous admixture, not replacing acids but bases, and most frequently[61]
alumina (Al2O3), for as a rule the amount of alumina decreases
as that of the boric anhydride increases in them. This substitution
is explained by the similarity between the atomic composition
of the oxides of aluminium (alumina) and boron. The subdivision of
oxides into basic and acid can in no way be sharply defined, and here
we meet with the most conclusive proof of the fact, for the oxides of
boron and aluminium belong to the number of intermediate oxides,
closely approaching the limit separating the basic from the acid oxides.
Their type (Chapter XV.) R2O3 is intermediate between those of the
basic oxides R2O and RO and those of the acid oxides R2O5 and RO3.
If we turn our attention to the chlorides, we remark that lithium
chloride is soluble in water, is not volatile, and is not decomposed by
water; the chlorides of beryllium and magnesium are more volatile,
and although not entirely, still are decomposed by water; whilst the
chlorides of boron and aluminium are still more volatile and are decomposed
by water. Thus the position of boron and aluminium in the series
of the other elements is clearly defined by their atomic weights, and
shows us that we must not expect any new and distinct functions in
these elements.
Boron was originally known in the form of sodium borate,
Na2B4O7,10H2O, or borax, or tincal, which was exported from Asia,
where it is met with in solution in certain lakes of Thibet; it has also
been discovered in California and Nevada, U.S.A.[1] Boric acid was
afterwards found in sea-water and in certain mineral springs.[2] Its[62]
presence may be discovered by means of the green coloration which it
communicates to the flame of alcohol, which is capable of dissolving free
boric acid.[3] Many of the boron compounds employed in the arts are
obtained from the impure boric acid which is extracted in Tuscany from
the so-called suffioni. In these localities, which present the remains
of volcanic action, steam mixed with nitrogen, hydrogen sulphide, small
quantities of boric acid, ammonia, and other substances, issue from the
earth.[3 bis] The boric acid partially volatilises with the steam, for if a
solution of boric acid be boiled, the distillate will always contain a certain
amount of this substance.[4]
[63]
If boric acid be introduced into an excess of a strong hot solution
of sodium hydroxide, then, on slowly cooling, the salt NaBO2,4H2O
crystallises out. This salt contains an equivalent of Na2O to one equivalent
B2O3. It might be termed a neutral salt did it not possess
strongly alkaline reactions and easily split up into the alkali and the
more stable borax or biborate of sodium mentioned above, which contains
2B2O3 to Na2O.[5] This salt is prepared by the action of boric[64]
acid on a solution of sodium carbonate. Borax may be perfectly purified
by crystallisation. If a saturated and hot solution of borax be mixed
with strong hydrochloric acid, common salt and a normal crystalline
hydrate of boric acid are formed. The composition of this hydrate
is B(HO)3, according to the form BX3—that is, of the composition
B2O3,3H2O. This is the easiest method of obtaining pure boric acid.
The water is easily expelled from this hydrate; it loses half at 100° and
the remainder on further heating, and the remaining B2O3 or boric
anhydride fuses at 580° (according to Carnelley), forming at first a
ductile (easily drawn out into threads), tenacious mass and then a
colourless liquid solidifying to a transparent glass, which absorbs
moisture from the atmosphere and then becomes cloudy.[6] Only the[65]
alkaline salts of boric acid are soluble in water, but all borates are
soluble in acids, owing to their easy decomposability and the solubility
of boric acid itself. Although boric anhydride, B2O3, absorbs 3H2O
from damp air, still in the presence of water it always[7] combines with
a less quantity of bases (borax only contains 1⁄6). However, fused boric
anhydride forms a crystalline compound with magnesium of the same
type as the hydrate (MgO)3B2O3 (Ebelmann), and even with sodium it
forms (Na2O)3B2O3 or Na3BO3 (Benedict). As a rule, the salts of
boric acid contain less base, although they are all able to form saline
compounds with bases when fused. Generally, vitreous fluxes are
formed by this means,[8] which when fused recall ordinary aqueous solutions
in many respects. Some of them crystallise on solidifying, and
then they have, like salts, a definite composition. The property of
boric anhydride of forming higher grades of combination with basic
oxides when fused explains the power of fused borax to dissolve metallic
oxides, and the experiments of Ebelmann on the preparation of artificial
crystals of the precious stones by means of boric anhydride. Boric
anhydride is, although with difficulty, volatile at a high temperature,
and therefore if it dissolves an oxide, it may be partially driven off from
such a solution by prolonged and powerful ignition; in which case
the oxides previously in solution separate out in a crystalline form,
and frequently in the same forms as those in which they occur in
nature—for example, crystals of alumina, which by itself fuses with
difficulty, have been obtained in this manner. It dissolves in
molten boric anhydride, and separates out in natural rhombohedric
crystals. In this way Ebelmann also obtained spinel—that is, a[66]
compound of magnesium and aluminium oxides which occurs in
nature.[9]
Free boron was obtained (1809) by Davy, Gay-Lussac, and Thénard
when they obtained the metals of the alkalis, for boric anhydride
when fused with sodium gives up its oxygen to the sodium, and free
boron is liberated as an amorphous powder like charcoal.[10] It is of a
brown colour, specific gravity 2·45 (Moissan), and when dry does not
alter in the air at the ordinary temperature; but it burns when ignited
to 700°, and in so doing combines not only with the oxygen of the air, but
also with the nitrogen. However, the combustion is never complete,
because the boric anhydride formed on the surface covers the remaining
mass of the boron, and so preserves it from the action of the
oxygen. Acids, even sulphuric (forming SO2) and phosphoric (forming
phosphorus), easily oxidise amorphous boron, especially when[67]
heated, converting it into boric acid. Alkalis have the same action
on it, only in this case hydrogen is evolved. Boron decomposes
steam at a red heat, also with evolution of hydrogen.
Amorphous boron, like charcoal, dissolves in certain molten metals.
The property of fused aluminium of dissolving boron in considerable
quantity is very striking; on cooling such a solution, the boron partially
combined with the aluminium separates out in a crystalline
form, and its properties are then exceedingly remarkable. The
crystalline boron may be obtained by heating (to 1,300°) the pulverulent
boron with aluminium in a well-closed crucible, the access of air
being prevented as far as possible. After cooling, crystals are observed
on the surface of the aluminium, and may easily be separated by dissolving
the latter in hydrochloric acid, which does not act on the crystals. The
specific gravity of the crystals is 2·68; they are partially transparent,
but are for the most part coloured dark brown; they contain about
4 p.c. of carbon and up to 7 p.c. of aluminium, so that they cannot
be considered as pure boron. Nevertheless, the properties of this
crystalline substance, which was obtained by Wöhler and Deville,
are very remarkable. It most closely resembles the diamond in its
properties—in fact, these crystals have the lustre and high refracting
power proper to the diamond only, whilst their hardness competes with
that of the diamond. Their powder polishes even the diamond, and like
the diamond scratches the sapphire and corundum. Crystalline boron is
much more stable with respect to chemical reagents than the amorphous
variety, and as it resembles the diamond, so amorphous boron, on the
other hand, distinctly recalls certain of the properties of charcoal; thus
a certain resemblance exists between boron and carbon in a free state,
which is further justified by the proximity of their positions in the
periodic system.
Among the other compounds of boron, those with nitrogen and
the halogens are the most remarkable. As already mentioned above,
amorphous boron combines directly with nitrogen at a red heat. If
it be heated in a glass tube in a stream of nitric oxide, perfect
combustion takes place, 5B + 3NO = B2O3 + 3BN. If the residue
be treated with nitric acid, the boric anhydride dissolves, whilst the
boron nitride remains[11] as an extremely light white powder, which[68]
is sometimes partially crystalline and greasy to the touch, like talc. It
is infusible and unchanged, even at the melting-point of nickel. In
general, it is remarkable for its great stability with respect to chemical
reagents. Nitric and hydrochloric acids, as well as alkaline solutions,
and hydrogen and chlorine at a red heat, have no action on it.
When fused with potash, it evolves ammonia, and when ignited in
steam it also yields ammonia: 2BN + 3H2O = B2O3 + 2NH3.[12]
No less remarkable is the compound of boron with fluorine—boron
fluoride, BF3. It is produced in many instances when compounds of
boron and of fluorine are brought together.[13] The most convenient
method of preparing it is by heating a mixture of calcium fluoride
with boric anhydride and sulphuric acid, 3CaF2 + B2O3 + 3H2SO4
= 3CaSO4 + 3H2O + 2BF3.[14] It is a colourless liquefiable gas (the
liquid boils at -100°), which on coming into contact with damp air
forms white fumes, owing to its combining with water. One volume
of water dissolves as much as 1,050 volumes of this gas (Bazaroff),
forming a liquid which disengages boron fluoride when heated, and
distils over unaltered. Boron fluoride chars organic matter, owing to its
taking up the water from it, and in this respect it acts like sulphuric
acid. The behaviour of boron fluoride with water must be understood as
a reversible reaction, since with water it yields hydrofluoric and boric
acids, whilst they, acting on one another, re-form boron fluoride and
water. A state of equilibrium is set up between these four substances
(and between two reversible reactions) which is distinctly dependent
on the mass of the water.[14 bis] When boron fluoride is in great excess,
the equilibrated system, which is capable of distilling over (sp. gr.[69]
of the liquid 1·77), has a composition BF3,2H2O (or B2O3,H2O,6HF).
It has also its corresponding salts.[15] It is a caustic liquid, having the
properties of a powerful acid; but it does not act on glass, which shows
that there is no free hydrofluoric acid present. Under the action of
water this system changes, with the formation of boric acid and
hydroborofluoric acid (HBF4) according to the equation 4BF3H4O2
= 3HBF4 + BH3O3 + 5H2O.[16] This hydroborofluoric acid has its
corresponding salts—for instance, KBF4. On evaporating the aqueous
solution this free acid decomposes, with the evolution of hydrofluoric
acid, and a stable system is again obtained: 2HBF4 + 5H2O
= B2F6H10O5 + 2HF. The resultant solution (containing 2BF3,5H2O,
sp. gr. 1·58), which is identical with that formed by the evaporation of
a solution of boric acid with hydrofluoric acid, again only contains
a compound of boron fluoride with water. Probably there are
various other possible and more or less stable states of equilibrium
and definite compounds of boron fluoride, hydrofluoric acid, and
water.
Nothing of this kind occurs with boron chloride, because hydrochloric
acid does not act on boric acid. However, amorphous boron
at 400° burns in chlorine, and at 410° forms boron chloride, BCl3.
The boron burns in the chlorine, forming a gas which, in a freezing
mixture, condenses into a liquid boiling at 17°, and gives up its excess
of chlorine, if there be any, to mercury. The specific gravity of this
liquid is 1·42 at 6°. Boron chloride may also be directly obtained
from boric anhydride by the simultaneous action of charcoal and
chlorine at a high temperature: B2O3 + 3C + 3Cl2 = 2BCl3 + 3CO. It
is also obtained by the action of phosphoric chloride on boric anhydride
in a closed tube at 200° It is completely decomposed by
water, like the chloranhydride of an acid, boric acid being formed;
hence it fumes in the air: 2BCl3 + 6H2O = 2BH3O3 + 6HCl. Boron[70]
forms with bromine a similar compound, BBr3, specific gravity at
6° = 2·64, boiling at 90°. The vapour densities of the fluoride, chloride,
and bromide of boron show that they contain three atoms of the
halogen in the molecule—that is, that boron is a trivalent element
forming BX3.[16 bis]
As in the first group lithium is followed by sodium, giving a more
basic oxide, so in the second group beryllium is followed by magnesium,
and so also in the third group there is, besides the lightest element,
boron, whose basic character is scarcely defined, aluminium, Al = 27,
whose oxide, alumina, has somewhat distinct basic properties, which,
although not so powerful as in magnesium oxide, are more distinct
than in boric anhydride. Among the elements of the third group,
aluminium is the most widely distributed in nature; it will be sufficient
to mention that it enters into the composition of clay to demonstrate
the universal distribution of aluminium in the earth's crust.
Alumina is so named from its being the metal of alums (alumen).
Clay, which is so widely distributed and familiar to everybody, is
the insoluble residue obtained after the action of water containing
carbonic acid on many rocks, and especially on the felspars contained
in some of them. Felspar is a compound containing potash or soda,
alumina, and silica. The primary rocks, like granite, contain many
similar compounds (see Chapter XVIII.: Felspars). Felspar is acted
on by water containing carbonic acid, all the alkalis (potash and
soda), and a portion of the silica passing into the water as substances
which are soluble and carried away by it, whilst the alumina and
silica left from the felspar remain on the spot where the solution
has taken place. This is the original method of the formation of
clay in its primary deposits among rocks along whose crevices the
atmospheric water has permeated. Such primary deposits often contain
a white pure clay, termed kaolin or porcelain clay. But such clay is a
rarity, because the conditions for its formation are rarely met with.
The water, whilst acting chemically on rocks, at the same time destroys
them mechanically, and carries off the finely divided residues of disintegration
with it. Clay is most easily subjected to this mechanical
action of water, because it is composed of grains of exceedingly small
size and void of any visible crystalline structure, which easily remain[71]
suspended in water. The cloudy water of running mountain
streams generally contains particles of clay in suspension, owing to the
above-described chemical and mechanical action of the water on the
minerals contained in the mountain rocks. Together with these
minute particles of clay the water carries away the coarser components
on which it is not able to act—for example, splinters of rock,
grains of mica, quartz, &c. They were originally held together by
those minerals which form clay. When the water acts on these
binding minerals, a sandy mass is formed which water bears away.
The cloudy water in which the particles of clay and sand are held
in suspension carries them to, and deposits them at, the estuaries of
rivers, lakes, seas, and oceans. The coarser particles are first deposited
and form sand and similar disintegrated rocky matter, whilst the
clay, owing to its finely divided state, is carried on further, and is
only deposited in the still parts of the rivers, lakes, &c. Such disintegrations
of rocks and separations of clay from sand have been
gradually going on during the millions of years of the earth's existence,
and are now proceeding, and have been the cause of the formation of
the immense deposits of sandstone and clay now forming a part of the
earth's strata. Such beds of clay may have been transferred by currents
and streams from one locality to another, so that we must distinguish
between primary and secondary deposits of clay. In places
these beds of clay have, owing to long exposure under water, and
perhaps partially owing to the action of heat, undergone compression,
and have formed the rocky masses known as clay slates and schists,
which sometimes form entire mountains. Roofing slates belong to this
class of rocks.
From what has been said above it will be evident that these
deposits can never consist of a chemically pure and homogeneous substance,
but will contain all kinds of extraneous insoluble finely divided
matter, and especially sand—that is, fragments of rock, chiefly quartz
(SiO2). It is, however, possible to considerably purify clay from
these impurities, owing to the fact that they are the result of
mechanical disintegration, whilst the clay has been formed as a residue
of the chemical alteration of rocky matter, and therefore its particles
are incomparably more minute than the particles of sand and other
rock fragments mixed with it. This difference in the size of the grains
causes the clay to remain longer in suspension when shaken up in water
than the coarser grains of sand. If clay be shaken up in water, and
especially if it be previously boiled in it, and if after the first portion
has settled the cloudy water be decanted, it will give a deposit of a
very much purer clay than the original. This method is employed for[72]
purifying kaolin designed for the manufacture of the best kinds of
china, earthenware, &c. A similar method is also employed in the investigation
of earths for determining the composition of soils chiefly
composed of a mixture of sand, clay, limestone, and mould. The
limestone is soluble in dilute acids, but neither the clay nor sand passes
into solution by this means, and therefore the limestone is easily
separated in the investigation of soils. The clay is separated from the
sand by a mechanical method similar to that described above, and
termed levigation.[17]
[73]
By treating clay with strong sulphuric acid, which dissolves the
alumina in it, and then (by means of an alkaline carbonate) dissolving the
silica which was combined with the alumina in the clay (but not that[74]
occurring in the form of sand, &c., which is hardly dissolved by carbonate
of soda solution at all even on boiling), we may form an idea of
the proportion between the component parts of a clay; and by igniting
it at a high temperature, we may determine the amount of water held
in it. In the purer sorts of clay dried at 100° (sp. gr. of pure kaolin is
about 2·5) this proportion is about 2SiO2 : 2H2O : Al2O3. In this case
the conversion of felspar into kaolin is expressed by the equation:—
| K2O,Al2O3,6SiO2 |
= |
Al2O3,2SiO2 |
+ |
K2O,4SiO2; |
| Felspar |
|
Kaolin |
|
|
the compound K2O,4SiO2 passes into solution.
But as a rule clays contain from 45 to 60 p.c. of silica, from 20 to
30 p.c. of alumina, and about 12 p.c. of water; and it cannot be
supposed that clays are always homogeneous, because they are
an aggregation of residues (of silico-aluminous compounds) which
are unacted on by water. Nevertheless, clays always contain a
hydrous compound of alumina and silica, which is able to give up the
alumina contained by it as a base to strong sulphuric acid, forming
aluminium sulphate, which is soluble in water. After this treatment
the silica remains, and is soluble in a solution of an alkaline carbonate.[18]
[75]
Clay is the source from which alumina, Al2O3, and the majority of
the compounds of aluminium are prepared. Among these compounds
the most important are the alums—that is, the double sulphates of
potassium (and allied metals) and aluminium, AlK(SO4)2,12H2O. When
clay is treated with sulphuric acid diluted with a certain amount of
water, aluminium sulphate, Al2(SO4)3, is formed; and if potassium
carbonate or sulphate be added to this solution, a double salt or
alum is obtained in solution. The alums crystallise easily, and are
prepared on a very large manufacturing scale owing to their being
employed in the process of dyeing. Alums are soluble in water, and,
on the addition of ammonia to their solutions, they give hydrous
alumina, or aluminium hydroxide, as a white gelatinous precipitate,
which is insoluble in water but easily soluble in acids, even when dilute,
and in aqueous soda or potash. The solubility of alumina in acids
indicates the basic character of the oxide, and its solubility in alkalis
and its power of forming compounds with them shows the weakness
of this basic character. However, the feeblest acids, even carbonic
acid, take up the alkali from such a solution, and the alumina then
separates out in a precipitate as the hydroxide. It must also be remembered
as characteristic of the salt-forming properties of alumina
that it does not combine with such feeble acids as carbonic, sulphurous,
or hypochlorous, &c.—that is, its compounds with these acids are
decomposed by water. It is also important to observe that the
hydroxide is not soluble in aqueous ammonia.
Alumina, Al2O3—that is, the anhydrous aluminium oxide—is
met with in nature, sometimes in a somewhat pure state, having
crystallised in transparent crystals, which are often coloured by impurities
(chromic, cobaltic, and ferric compounds). Such are the ruby
and sapphire, the former red and the latter blue. They have a specific
gravity 4·0, are distinguished by their very great hardness, which is
second only to that of the diamond, and they represent the purest
form of alumina. They are found in Ceylon and other islands of the
Indian Archipelago, embedded in a rock matrix.[18 bis] Corundum is the[76]
same crystallised anhydrous alumina coloured brown by a trace of
oxide of iron. A very much larger portion of this impurity occurs in
emery, which is found in crystalline masses in Asia Minor and in
Massachusetts, and owing to its extreme hardness is employed for
polishing stones and metals. In this anhydrous and crystalline state
the aluminium oxide is a substance which very powerfully resists the
action of reagents, and is insoluble both in solutions of the alkalis
and in strong acids. It is only capable of passing into solution after
being fused with alkalis.[19] Alumina may be obtained in this form by
artificial means if the hydroxide be ignited and then fused in the oxyhydrogen
flame.[20] Alumina also occurs in nature in combination with
water—as, for instance, in the rather rare minerals hydrargillite
(sp. gr. 2·3), Al2O3,3H2O = 2Al(HO)3, and diaspore, Al2O_3,H2O
= 2AlO(HO) (sp. gr. 3·4). A less pure hydrate, mixed with ferric
oxide, sometimes occurs in masses (at Baux in the south of France) and
is termed bauxite; it contains Al2O3,2H2O = Al2O(HO)4 (sp. gr. 2·6).
When bauxite is ignited with sodium carbonate, carbonic anhydride
is liberated and the alumina then combines with the sodium oxide,
forming a saline aluminate of the oxides of aluminium and sodium.
This is taken advantage of in practice for the preparation of pure
alumina compounds on a large scale, for bauxite is found in large
masses (in the South of France, in Austria, and in Carolina in South
America), and the resultant compound of alumina and sodium
is soluble in water and does not contain ferric oxide. This solution
when subjected to the action of carbonic anhydride gives a precipitate
of aluminium hydroxide,[21] which with acids forms aluminium[77]
salts. If aqueous ammonia be added to a solution of aluminium
sulphate a gelatinous precipitate is formed, which at first remains
suspended in the liquid and then on settling forms a gelatinous mass,
which itself indicates the colloidal property of aluminium hydroxide.
The following points are characteristic of this colloidal state: (1) in an
anhydrous state such a colloidal substance is insoluble in water, as
alumina is; (2) in the hydrated state, it is gelatinous and insoluble in
water; and (3) it is also capable of existing in solutions, from which
it separates out in a non-crystalline state, forming a substance resembling
glue. These different states of colloids were distinguished by
Graham, who gave them the following very characteristic names. He
called the gelatinous form of the hydrate hydrogel, i.e. a gelatinous
hydrate, and the soluble form of the aqueous compound, hydrosol,
from the Latin for a soluble hydrate. Alumina readily and frequently
assumes these states. The gelatinous hydrate of alumina is its
hydrogel. It is, as has been already mentioned, insoluble in water,
and, like all similar hydrogels, shows not the faintest sign of crystallisation;
it is apt to vary in many of its properties with the amount of
water it contains, and loses its water on ignition, leaving a white
powder of the anhydrous oxide. The hydrogel of alumina is soluble
both in acids and alkalis. It may also be obtained by the evaporation
of its solutions in such feebly energetic acids as volatile acetic acid.
These properties are very frequently made use of in the arts, and
especially in the processes of dyeing, because the hydrogel of alumina
in precipitating attracts a number of colouring matters from their
solutions, the precipitate being thus coloured by the dyes attracted.[22][78]
The preparation of fixed dyes and the employment of aluminous
compounds (mordants) in the processes of dyeing are founded on this
fact.[23] When precipitated upon the fibres of tissues (calicoes, linens,
&c.) the aluminium hydroxide renders them impermeable to water;[79]
this may be taken advantage of for the preparation of waterproof
tissues.
The hydrosol of alumina—i.e. the soluble aluminium hydroxide—is
more difficult to obtain.[24] In order to obtain this soluble variety of
alumina, Graham took a solution of its hydrogel in hydrochloric acid—that
is, a solution of aluminium chloride, which is able to dissolve a
still further quantity of the hydrogel of alumina, forming a basic salt
having probably one of the compositions Al(HO)Cl2 or Al(HO)2Cl.
When such a solution, considerably diluted with water, is subjected to
dialysis—that is, to diffusion through a membrane[25]—the hydrochloric
acid diffuses through the membrane and leaves the alumina in the
form of hydrosol. The resultant solution, even when only containing
two or three per cent. of alumina, passes into the hydrogel state with
such facility that it is sufficient to transfer it from one vessel to
another which has not been previously washed with water, for the
entire mass to solidify into a jelly. But a solution containing not
more than one-half per cent. of alumina may even be boiled without
coagulating; however, after the lapse of several days this solution will
of its own accord yield the hydrogel of alumina.[25 bis]
[80]
With respect to alumina as a base, it is very important to observe
that it is not only capable of combining with other bases[26] but that
it does not give salts with feeble volatile acids (like carbonic and
hypochlorous); it forms salts which are easily decomposed by water,
especially when heated,[27] as well as double and basic salts,[28] so that it
forms a clear example of a feeble base.[29] To these characteristics of
alumina we must add that it not only gives compounds of the type
AlX3, but also the polymeric type Al2X6, even when X is a simple
univalent haloid like chlorine. Deville and Troost showed (1857)
that the vapour density of aluminium chloride (at about 400°) is 9·37
with respect to air—that is, nearly 135 with respect to hydrogen, and
therefore the formula of its molecule is expressed by Al2Cl6, and
not AlCl3,[30] although in the case of boron, arsenic, and antimony,[81]
which give oxides R2O3 of the same composition as Al2O3, the
chlorine compounds form non-polymeric molecules, BCl3, AsCl3,[82]
SbCl3.[31] This duplication (polymerisation) of the form AlX3 is connected
with the facility with which the salts of aluminium combine
with other salts to form double salts and with aluminium hydroxide
itself to form basic salts.
Aluminium sulphate, Al2(SO4)3, which is obtained by treating clay
or the hydrates of alumina with sulphuric acid, crystallises in the cold
with 27H2O, or at the ordinary temperature in pearly crystals, which
are greasy to the touch and contain 16H2O.[32] Its solutions act like sulphuric
acid—for instance, they evolve hydrogen with zinc, forming
basic salts, which are sometimes met with in nature (aluminite,
Al2O3,SO3,9H2O, alumiane, Al2O3,2SO3, and others), and may be obtained
by the decomposition of normal salts and by the direct solution of
the hydroxide in normal salts: these exhibit a varying composition,
(Al2O3)n(SO3)m(H2O)q, where m/n is less than 3. Aluminium sulphate
is now prepared (from the pure hydrate obtained from bauxite, Note
21) in large quantities for dyeing purposes (instead of alums) as a
mordant. With solutions of the alkali sulphates (potassium, sodium,
ammonium, rubidium, and cæsium sulphates), the normal salt easily
forms double salts, termed alums—for example, the ordinary crystalline
alum contains KAl(SO4)2,12H2O, or K2SO4,Al2(SO4)3,24H2O. In the
ammonium alums (which leave a residue of alumina when ignited)
the potassium is replaced by ammonium (NH4). Alums are used
in large quantities, because there is scarcely any other salt which
crystallises so easily. In this respect the alums formed by potassium
and ammonium are equally convenient to purify, because they present
a considerable difference in their solubility at the ordinary
and higher temperatures. If the crystallisation be conducted rapidly,
the salt separates in minute crystals, but if it be slowly deposited,
especially in large masses, as in factories, then crystals several centimetres
long are sometimes obtained. At a higher temperature alums are very
much more soluble, and crystallise with greater difficulty, and are therefore
less easily freed from impurities; at 0° 100 parts of water dissolve
3 parts, at 30° 22 parts, at 70° 90 parts, and at 100° 357 parts of
potassium alum.[33] The solubility of ammonium alum is slightly less.[83]
The specific gravity of potassium alum is 1·74, of ammonium alum
1·63, and of sodium alum 1·60. Alums easily part with their water of
crystallisation; thus potash alum partially effloresces when exposed to
the air, and loses 9 mol. H2O under the receiver of an air-pump. At
100°, dry air passed over alums takes up nearly all their water. As
we have already mentioned (Chapter XV.), the law of isomorphous substitutions
exhibits itself more clearly in the alums than in any other
salts, and all alums not only contain the same amount of water of
crystallisation, MR(SO4)2,12H2O (where M = K, NH4, Na; R = Al,
Fe, Cr), and appear in crystals whose planes are inclined at equal
angles, but they also give every possible kind of isomorphous mixture.
The aluminium in them is easily replaced by iron, chromium, indium
and sometimes by other metals, whilst the potassium may be substituted
by sodium, rubidium, ammonium, and thallium, and the
sulphuric acid may be replaced by selenic and chromic acids.
Aluminium chloride, Al2Cl6, is obtained, like other similar chlorides,
(for instance MgCl2) either directly from chlorine and the metal, or by heating
to redness an intimate mixture of the amorphous anhydrous oxide and
charcoal in a stream of dry chlorine.[33 bis] The resultant sublimate is very
volatile,[34] and forms a crystalline, easily fusible mass, which deliquesces
in the air and easily dissolves in water, with the evolution of a large[84]
amount of heat.[34 bis] On evaporating this solution, hydrochloric acid
and aluminium hydroxide are liberated. But if the solution be
heated in a closed tube, with an excess of hydrochloric acid, then, on
cooling, crystals of AlCl3,6H2O are obtained—that is, aluminium
chloride both combines with water and is decomposed by it. And the
faculty of the type AlX3 for combining with other molecules is seen in
the compounds of AlCl3 with many other chlorine compounds. Thus,
for example, a mixture of aluminium chloride with sulphur tetrachloride
gives Al2Cl6,SCl4, under the action of chlorine, whilst with phosphorus
pentachloride it forms AlCl3,PCl5; it also combines with NOCl. Thus,
the compounds AlCl3,NOCl, AlCl3,POCl3, AlCl3,3NH3, AlCl3,KCl,
AlCl3,NaCl are known.[35] The compound of aluminium and sodium
chlorides, AlNaCl4, is very fusible and much more stable in the air
than aluminium chloride itself. It seems to be of the same type as
the alums. This compound, AlNaCl4, is employed in the extraction
of metallic aluminium, as we shall presently proceed to describe.[85]
Aluminium bromide, which is obtained by the direct combination of
metallic aluminium with bromine, closely resembles the chloride; it
melts at 90°, volatilises at 270°, and its vapour density indicates the
formula Al2Br6. Aluminium iodide is obtained by heating iodine with
finely divided aluminium in a closed tube; it is so easily decomposed
by oxygen that its vapour even explodes when mixed with it.[36]
Metallic Aluminium was first prepared by Wöhler in 1822 as
a grey powder by the action of potassium on aluminium chloride.
He afterwards (in 1845) obtained it as a white compact metal,
unoxidisable in the air, and only slowly attacked by acids. Owing
to the vast and wide occurrence of clay, many efforts have been made
in investigating in detail the methods for the extraction of this metal.
These efforts were brought to a successful issue (1854) by Sainte-Claire
Deville, who is also renowned for his doctrine of dissociation. Experiments
on a large scale have proved that metallic aluminium, although
possessed of great lightness, strength, and durability, is not so generally
suitable for technical purposes as was at first thought. Nitric and many
other acids, indeed, do not act on it, but the alkalis, alkaline substances,
and even salts—for instance, moist table salt—humidity, &c.,[36 bis] tarnish
it, and hence objects made of aluminium suffer at the surfaces, alter,
and cannot, as was hoped, replace the precious metals, from which it
differs in its extreme lightness. But the alloys made with aluminium
(especially with copper, for example aluminium bronze) are very
valuable in their properties and applications.
The Deville method for the preparation of metallic aluminium is[86]
based on the decomposition of the above-mentioned compound of
sodium and aluminium chlorides by metallic sodium. The compound
is obtained by passing the vapour of aluminium chloride (evolved from
a mixture of alumina, extracted from bauxite or cryolite, with
charcoal ignited in a stream of chlorine) over red-hot salt, when the
compound AlNaCl4, is itself volatilised, and may in this manner be
obtained pure. A mixture of this compound with salt and fluor spar,
or with cryolite, is heated with a certain excess of sodium, cut into
small lumps. On a large scale this operation is carried on in special
furnaces with a small access of air and at a high temperature. The decomposition
takes place chiefly according to the equation NaAlCl4 + 3Na
= 4NaCl + Al. Neither charcoal nor zinc will reduce the oxygen
compounds of aluminium; even sodium and potassium do not act on
alumina. Moreover, metallic aluminium, like magnesium, is able to
reduce even the metals of the alkalis from their oxygen compounds.
This is connected with the fact that the atom of oxygen evolves
more heat in combining with Al (and Mg) than it does in combining with
other metals; whilst on the other hand, chlorine (and the other halogens)
evolve more heat in combining with the metals of the alkalis.[36 tri]
Since the close of the eighties the metallurgy of aluminium has
taken a new direction, based upon the action of an electric current
upon cryolite at a high temperature,[37] and the solution of oxide of
aluminium (obtained from bauxite or in the form of corundum) in
it; under these conditions metallic aluminium is reduced at the
negative pole (cathode) in a sufficiently pure state, and if the cathode
be copper, forms alloys with it. Such are Hall's and Cowle's (both in
the United States) and the Neuhausen process (where the current is
obtained from a dynamo worked by the Falls of the Rhine at Schaffhausen).[87]
As an example, we will describe (in the words of Prof. D. P.
Konovaloff, who became acquainted with this process at the Chicago
Exhibition), Hall's process as applied near Pittsburg, where it gives
about 1,500 kilos of Al a day. An iron box (about 1 metre long and
½ metre wide), provided with a well rammed down charcoal lining, is
charged with a mixture of cryolite and Al2O3 (from bauxite), over
which salt is strewn, and a current of 5,000 ampères at 20 volts is
passed through the mixture. The anode is composed of a carbon cylinder
(about 9 cm. in diameter), while the charcoal lining forms the cathode.
When the temperature inside the box is raised to a red heat by the
current, the mixture fuses and the Al2O3 begins to decompose. The
Al liberated collects at the bottom of the box, whilst the oxygen
evolved burns the charcoal anode. When the decomposition is at
an end, and the resistance of the mass increases, a fresh quantity of
Al2O3 is added, and this is continued until the amount of impurities
accumulated in the furnace and passing into the metal becomes too
great.[37 bis]
Aluminium has a white colour resembling that of tin—that is, it is
greyer than silver and has the feebly dull lustre of tin, but compared to
tin and pure silver, aluminium is very hard. Its density is 2·67—that
is, it is nearly four times lighter than silver and three times lighter
than copper. It melts at an incipient red heat (600°), and in so doing
is but slightly oxidised. At the ordinary temperature it does not alter
in the air, and in a compact mass it burns with great difficulty at a
white heat, but in thin sheets, into which it may be rolled, or as a very
fine wire, it burns with a brilliant white light, since it forms an infusible
and non-volatile oxide. Aluminium itself is non-volatile at a
furnace heat. These properties render Al a very good reducing agent,
and N. N. Beketoff showed that it reduces the oxides of the alkali
metals (Chapter XIII., Note 42 bis). Dilute sulphuric acid has scarcely
any action on it, but the strong acid dissolves it, especially with the aid
of heat. Nitric acid, dilute or strong, has no action whatever on it. On
the other hand, hydrochloric acid dissolves aluminium with great ease,[88]
as do also solutions of caustic soda and potash. In the latter cases
hydrogen is evolved.[38]
Aluminium forms alloys with different metals with great ease.
Among them the copper alloy is of practical use. It is called
aluminium bronze. This alloy is prepared by dissolving 11 p.c.
by weight of metallic aluminium in molten copper at a white
heat. The formation of the alloy is accompanied by the development
of a considerable quantity of heat, so that it glows to a bright white
heat. This alloy, which corresponds with the formula AlCu3, presents
an exceedingly homogeneous mass, especially if perfectly pure
copper be taken. It is distinguished for its capacity to fill up the
most minute impressions of the mould into which it may be cast,
and by its extraordinary elasticity and toughness, so that objects cast
from it may be hammered, drawn, &c., and at the same time it is fine-grained
and exceedingly hard, takes an excellent polish, and, what is
most important, its surface then remains almost unchangeable in the
air, and has a colour and lustre which may be compared to that of gold
alloys. Hence aluminium bronze is much used in the arts for making
spoons, watches, vessels, forks, knives, and for ornaments, &c. No
less important is the fact that the admixture of one-thousandth part
of aluminium with steel renders its castings homogeneous (free from
cavities) to an extent that could not be arrived at by other means, nor
does the quality of the steel in any respect deteriorate by this admixture,
but rather is it improved. In a pure state, aluminium is only
employed for such objects as require the hardness of metals with
comparative lightness, such as telescopes and various physical apparatus
and small articles.
According to the periodic system of the elements, the analogues of
magnesium are zinc, cadmium, and mercury in the second group. So
also in the third group, to which aluminium belongs, we find its corresponding
analogues gallium, indium, and thallium. They are all three[89]
so rarely and sparingly met with in nature that they could only be
discovered by means of the spectroscope. This fact shows that they
are partially volatile, as should be the case according to the property
of their nearest neighbours, the very volatile zinc, cadmium and mercury.
As with them, in gallium, indium, and thallium the density of
the metal, decomposability of compounds, &c., rises with the atomic
weight. But here we find a peculiarity which does not exist in the
second group. In the latter, the fusibility increases with the atomic
weight of magnesium, zinc, cadmium, and mercury; indeed, the
heaviest metal—mercury—is a liquid. In the third group it is not so.
In order to understand this it is sufficient to turn our attention to
the elements of the further groups of the uneven series—for instance,
to group V., containing phosphorus, arsenic, and antimony, or to
group VI., with sulphur, selenium, and tellurium, and also to
group VII., where chlorine, bromine and iodine are situated. In
all these instances the fusibility decreases with a rise of atomic
weight; the members of the higher series, the elements of a high
atomic weight, fuse with greater difficulty than the lighter elements.
The representatives of the uneven series of group III., aluminium,
gallium, indium, thallium, forming, as they do, a transition, all show
an intermediate behaviour. Here the most fusible of all is the medium
metal gallium,[38 bis] which fuses at the heat of the hand; whilst indium,
thallium, and aluminium fuse at much higher temperatures.
Zinc (group II.), which has an atomic weight 65, should be followed
in group III. by an element with an atomic weight of about 69. It
will be in the same group as Al and should consequently give R2O3,
RCl3, R2(SO4)3, alums and similar compounds analogous to those of
aluminium. Its oxide should be more easily reducible to metal than
alumina, just as zinc oxide is more easily reduced than magnesia. The
oxide R2O3 should, like alumina, have feeble but clearly expressed
basic properties. The metal reduced from its compounds should have a
greater atomic volume than zinc, because in the fifth series, proceeding
from zinc to bromine, the volume increases. And as the volume of
zinc = 9·2, and of arsenic = 18, that of our metal should be
near to 12. This is also evident from the fact that the volume of
aluminium = 11, and of indium = 14, and our metal is situated in
group III., between aluminium and indium. If its volume = 11·5[90]
and its atomic weight be about 69, then its density will be nearly 5·9.
The fact that zinc is more volatile than magnesium gives reason for
thinking that the metal in question will be more volatile than
aluminium, and therefore for expecting its discovery by the aid of
the spectroscope, &c.
These properties were indicated by me for the analogue of aluminium
in 1871, and I named it (see Chapter XV.) eka-aluminium.
In 1875, Lecoq de Boisbaudran, who had done much work in spectrum
analysis, discovered a new metal in a zinc blende from the Pyrenees
(Pierrefitte). He recognised its individuality and difference from
zinc, cadmium, indium, and the other companions of zinc by means of
the spectroscope; but he only obtained some fractions of a centigram
of it in a free state. Consequently only a few of its reactions were
determined, as, for instance, that barium carbonate precipitates the new
oxide from its salts (alumina, as is known, is also precipitated). Lecoq
de Boisbaudran named the newly discovered metal gallium. As one
would expect the same properties for eka-aluminium as were observed
in gallium, I pointed out this fact at the time in the Memoirs of the
Paris Academy of Sciences. All the subsequent observations of Lecoq
de Boisbaudran confirmed the identity between the properties of gallium
and those indicated for eka-aluminium. Immediately after this the
ammonium alum of gallium was obtained, but the most convincing proof
of all was found in the fact that the density of gallium although
first apparently different (4·7) from that indicated above, afterwards,
when the metal was carefully purified from sodium (which was first
used as a reducing agent), proved to be just that (5·9) which would
have been looked for in the analogue of aluminium; and, what
was very important, the equivalent (23·3) and atomic weight (69·8)
determined by the specific heat (0·08) were shown by experiment
to be such as would be expected. These facts confirmed the
universality and applicability of the periodic system of the elements.
It must be remarked that previous to it there was no means of either
foretelling the properties or even the existence of undiscovered
elements.[39]
Much more light has been thrown on that element of the aluminium[91]
group which follows after cadmium (its position in the periodic system
is III., 7, that is, it is in group III. in the 7th series). This is
indium, In, which also occurs in small quantities in certain zinc ores.
It was discovered (1863) by Reich and Richter (and more fully investigated
by Winkler) in the Freiberg zinc ores, and was named indium
from the fact that it gives to the flame of a gas-burner a blue coloration,
owing to the indigo blue spectral lines proper to it. The equivalent
(see Chapter XV., Note 15), specific heat, and other properties
of the metal confirm the atomic weight In = 113.[40]
Inasmuch as we found among the analogues of magnesium in
group II. a metal, mercury, heavier and more easily reduced than
the rest, and giving two grades of oxidation, so we should expect to
find a metal among the analogues of aluminium in group III. which
would be heavy, easily reduced, and give two grades of oxidation, and
would have an atomic weight greater than 200. Such is thallium.
It forms compounds of a lower type, TlX, besides the higher unstable
type TlX3, just as mercury gives HgX2 and HgX. In the form of
the thallic oxide, Tl2O3, the base is but feebly energetic, as would be
expected by analogy with the oxides Al2O3, Ga2O3, and In2O3, whilst
in thallous oxide, Tl2O, the basic properties are sharply defined,
as might be expected according to the properties of the type R2O
(Chapter XV.). Thallium was discovered in 1861 by Crookes and by
Lamy in certain pyrites. When pyrites are employed in the manufacture
of sulphuric acid, they are burned, and give besides sulphurous
anhydride the vapours of various substances which accompany the
sulphur, and are volatile. Among these substances arsenic and
selenium are found, and together with them, thallium. These substances
accumulate in a more or less considerable quantity in the[92]
tubes through which the vapours formed in the combustion of the
pyrites have to pass. When the methods of spectrum analysis were
discovered (1860), a great number of substances were subjected to
spectroscopic research, and it was observed that those sublimations
which are obtained in the combustion of certain pyrites contained an
element having a very sharply-defined and characteristic spectrum—namely,
in the green portion of the spectra it gave a well-defined band
(wave-length 535 millionth millimetres) which did not correspond with
any then known element.[41]
Under the action of a galvanic current solutions of thallium salts
deposit the metal in the form of a heavy powder. It is of a grey colour
like tin, is soft like sodium, and has a metallic lustre. Its specific
gravity is 11·8, it melts at 290°, and volatilises at a high temperature.
When heated slightly above its melting point it forms an insoluble
(in water) higher oxide, Tl2O3, as a dark-coloured powder, generally
however accompanied by the lower oxide Tl2O, which is also black
but soluble in water and alcohol. This solution has a distinctly
alkaline reaction. This thallous oxide, melts at 300°, and is easily
obtained from the hydroxide TlHO by igniting it without access of
air (in the presence of air the incandescent thallous oxide partly passes
into thallic oxide). Thallous hydroxide, TlOH, crystallises with one
molecule H2O in yellow prisms which are very easily soluble in water.
Metallic thallium may be used for its preparation, as the metal in the
presence of water attracts oxygen from the air and forms the hydroxide.
But metallic thallium does not decompose water, although it gives
a hydroxide which is soluble in water.[41 bis] All the other data for the[93]
chemical and physical properties of thallium, of its two grades of oxidation
and of their corresponding salts, are expressed by the position
occupied by this metal in virtue of its atomic weight Tl = 204, between
mercury Hg = 200, and lead Pb = 206.
Gallium, indium, and thallium belong to the uneven series, and there
should be elements of the even series in group III. corresponding
with calcium, strontium, and barium in group II. These elements
should in their oxides R2O3 present basic characters of a more
energetic kind than those shown by alumina, just as calcium,
strontium, and barium give more energetic bases than magnesium, zinc,
and cadmium. Such are yttrium and ytterbium, which occur in a
rare Swedish mineral called gadolinite, and are therefore termed
the gadolinite metals. To these belong also the metal lanthanum,
which accompanies the two other metals cerium and didymium in
the mineral cerite, and it therefore belongs to the cerite metals. All
these metals and certain others accompanying them, give basic oxides
R2O3. At first their formula was supposed to be RO, but the
application of the periodic system required their being counted as
elements of groups III. and IV., which was also confirmed by
the determination of the specific heats of these metals,[42] and better[94]
still by the fact that Nilson and Clève, in their researches on the
gadolinite metals (1879), discovered that they contain a peculiar and
very rare element, scandium, which by the magnitude of its atomic
weight, Sc = 44, and in all its properties, exactly corresponds with the
metal (previously foretold on the basis of the periodic system) ekaboron,
whose properties were determined by taking the cerite and gadolinite
metals as forming oxides R2O3.[43]
[95]
[96]
The brevity of this work and the great rarity of the above-mentioned
elements will give me the right to exclude their description, all the
more as the principles of the periodic system enable many of their
properties to be foreseen, and as their practical uses (cerium oxalate is
used in medicine, and didymium oxide in the manufacture of glass, a
mixture of the oxides of lanthanum and similar metals is employed for[97]
[98]
giving a bright light, as this mixture emits a brilliant white light
when brought to incandescence) are very limited, by reason of their
great rarity in nature, and the difficulty of separating them from one
another.
[99]
CHAPTER XVIII
SILICON AND THE OTHER ELEMENTS OF THE FOURTH GROUP
Carbon, which gives the compounds CH4, and CO2, belongs to the
fourth group of elements. The nearest element to carbon is silicon,
which forms the compounds SiH4 and SiO2; its relation to carbon is
like that of aluminium to boron or phosphorus to nitrogen. As carbon
composes the principal and most essential part of animal and vegetable
substances, so is silicon almost an invariable component part of the
rocky formations of the earth's crust. Silicon hydride, SiH4, like CH4,
has no acid properties, but silica, SiO2, shows feeble acid properties
like carbonic anhydride. In a free state silicon is also a non-volatile,
slightly energetic non-metal, like carbon. Therefore the form and
nature of the compounds of carbon and silicon are very similar. In
addition to this resemblance, silicon presents one exceedingly important
distinction from carbon: namely, the nature of the higher degree of
oxidation. That is, silica, silicon dioxide, or silicic anhydride, SiO2 is
a solid, non-volatile, and exceedingly infusible substance, very unlike
carbonic anhydride, CO2, which is a gas. This expresses the essential
peculiarity of silicon. The cause of this distinction may be most
probably sought for in the polymeric composition of silica compared
with carbonic anhydride. The molecule of carbonic anhydride contains
CO2, as seen by the density of this gas. The molecular weight
and vapour density of silica, were it volatile, would probably correspond
with the formula SiO2, but it might be imagined that it would correspond
to a far higher atomic weight of SinO2n, principally from the
fact that SiH4 is a gas like CH4, and SiCl4 is a liquid and volatile,
boiling at 57°—that is, even lower than CCl4, which boils at 76°. In
general, analogous compounds of silicon and carbon have nearly the
same boiling points if they are liquid and volatile.[1] From this it might[100]
be expected that silicic anhydride, SiO2, would be a gas like carbonic
anhydride, whilst in reality silica is a hard non-volatile substance,[1 bis]
and therefore it may with great certainty be considered that in this
condition it is polymeric with SiO2, as on polymerisation—for instance,
when cyanogen passes into paracyanogen, or hydrocyanic acid into
cyanuric acid (Chapter IX.)—very frequently gaseous or volatile
substances change into solid, non-volatile, and physically denser and
more complex substances.[2] We will first make acquaintance with free
silicon and its volatile compounds, as substances in which the analogy
of silicon with carbon is shown, not only in a chemical but also in a
physical sense.[3]
[101]
Free silicon can be obtained in an amorphous or crystalline state.
Amorphous silicon is produced, like aluminium, by decomposing the
double fluoride of sodium and silicon (sodium silicofluoride) by means
of sodium: Na2SiF6 + 4Na = 6NaF + Si. By treating the mass thus
obtained with water the sodium fluoride may be extracted and the
residue will consist of brown, powdery silicon. In order to free it from
any silica which might be formed, it is treated with hydrofluoric acid.
This silicon powder is not lustrous; when heated it easily ignites, but
does not completely burn. It fuses when very strongly heated, and[102]
has then the appearance of carbon.[4] Crystalline silicon is obtained in
a similar way, but by substituting an excess of aluminium for the
sodium: 3Na2SiF6 + 4Al = 6NaF + 4AlF3 + 3Si. The part of the
aluminium remaining in the metallic state dissolves the silicon, and
the latter separates from the solution on cooling in a crystalline form.
The excess of aluminium after the fusion is removed by means of hydrochloric
and hydrofluoric acid. The best silicon crystals are obtained
from molten zinc; 15 parts of sodium silicofluoride are mixed with
20 parts of zinc and 4 parts of sodium, and the mixture is thrown
into a strongly heated crucible, a layer of common salt being used to
cover it; when the mass fuses it is stirred, cooled, treated with
hydrochloric acid, and then washed with nitric acid. Silicon, especially
when crystalline, like graphite and charcoal, does not in any way act
on the above-mentioned acids. It forms black, very brilliant, regular
octahedra having a specific gravity of 2·49; it is a bad conductor of
electricity, and does not burn even in pure oxygen (but it burns in
gaseous fluorine). The only acid which acts on it is a mixture of hydrofluoric
and nitric acids; but caustic alkalis dissolve in it like aluminium,
with evolution of hydrogen, thus showing its acid character. In
general silicon strongly resists the action of reagents, as do also boron
and carbon. Crystalline silicon was obtained in 1855 by Deville, and
amorphous silicon in 1826 by Berzelius.[4 bis]
Silicon hydride, SiH4, analogous to marsh gas was obtained first
of all in an impure state, mixed with hydrogen, by two methods: by
the action of an alloy of silicon and magnesium on hydrochloric acid,[5]
and by the action of the galvanic current on dilute sulphuric acid,
using electrodes of aluminium, containing silicon. In these cases[103]
silicon hydride is set free, together with hydrogen, and the presence
of the hydride is shown by the fact that the hydrogen separated
ignites spontaneously on coming into contact with the air, forming
water and silica. The formation of silicon hydride by the action
of hydrochloric acid on magnesium silicide is perfectly akin to the
formation of phosphuretted hydrogen by the action of hydrochloric
acid on calcium phosphide, to the formation of hydrogen sulphide by
the action of acids on many metallic sulphides, and to the formation
of hydrocarbons by the action of hydrochloric acid on white cast
iron. On heating silicon hydride—that is, on passing it through an
incandescent tube, it is decomposed into silicon and hydrogen, just like
the hydrocarbons, but the caustic alkalis, although without action
on the latter, react with silicon hydride according to the equation:
SiH4 + 2KHO + H2O = SiK2O3 + 4H2.
Silicon chloride, SiCl4, is obtained from amorphous anhydrous silica
(made by igniting the hydrate) mixed with charcoal,[6] heated to a white[104]
heat in a stream of dry chlorine—that is, by that general method by which
many other chloranhydrides having acid properties are obtained. Silicon
chloride is purified from free chlorine by distillation over metallic mercury.
Free silicon forms the same substance when treated with dry chlorine.
It is a volatile colourless liquid, which boils at 59° and has a specific
gravity of 1·52. It fumes strongly in air, has a pungent smell, and in
general has the characteristic properties of the acid chloranhydrides.
It is completely decomposed by water, forming hydrochloric acid and
silicic acid, according to the equation: SiCl4 + 4H2O = Si(OH)4 + 4HCl.[7]
[105]
The most remarkable of the haloid compounds of silicon is silicon
fluoride, SiF4. It is a gaseous substance only liquefied by intense cold,
-100°, and is obtained (Chapter XI.) directly by the action of hydrofluoric
acid on silica and its compounds (SiO2 + 4HF = 2H2O + SiF4), and
also by heating fluorspar with silica (2CaF2 + 3SiO2 = 2CaSiO3 + SiF4).[8]
In order to prepare silicon fluoride, sand or broken glass is mixed with
an equal quantity by weight of fluorspar and 6 parts by weight of
strong sulphuric acid, and the mixture is gently heated. It fumes
strongly in air, reacting with the aqueous vapours, although it is produced
from silica and hydrofluoric acid with the separation of water.
It is evident that a reverse reaction occurs here; that is to say,
the water reacts with the silicon fluoride, but the reaction is
not complete. This phenomenon is similar to that which occurs
when water decomposes aluminium chloride, but at the same time
hydrochloric acid dissolves aluminium hydroxide and forms the same
aluminium chloride. The relative amount of water present (together
with the temperature) determines the limit and direction of the
reaction. The faculty which silicon fluoride has of reacting with
water is so great that it takes up the elements of water from many
substances—for instance, like sulphuric acid, it chars paper. Water
dissolves about 300 volumes of this gas, but in this case it is not a
common dissolution which takes place, but a reaction. During the first
absorption of silicon fluoride by water, silicic acid is separated in the
form of a jelly, but a certain quantity of the silicon fluoride also
remains in the liquid, because the hydrofluoric acid formed dissolves
the other part of the silica[9] and forms the so-called hydrofluosilicic[106]
acid: H2SiF6 = SiF4 + 2HF = SiH2O3 + 6HF - 3H2O. That is to say,
a metasilicic acid, SiH2O3, in which O3 is replaced by F6. This
view of the composition of hydrofluosilicic acid may be admitted,
because it forms a whole series of crystallisable and well defined
salts. In general, the whole reaction of water on silicon fluoride may
be expressed by the equation: 3SiF4 + 3H2O = SiO(OH)2 + 2SiH2F6.
Hydrofluosilicic acid and silicic acid resemble each other as much, and
differ as much, in their chemical character as water and hydrofluoric
acid. For this reason silicic acid is a feebler acid than hydrofluosilicic
acid, and in addition to this the former is insoluble, and the latter
soluble, in water.[10] Hydrofluosilicic acid is also formed if silicic acid
be dissolved in a solution of hydrofluoric acid. It is incapable of
volatilising without decomposition, and on heating the concentrated
acid silicon fluoride is evolved, leaving an aqueous solution of hydrofluoric
acid. This is the reason why solutions of hydrofluosilicic acid
corrode glass. This decomposition may be further accelerated by the
addition of sulphuric acid, or even of other acids. Hydrofluosilicic acid,
when acting on potassium and barium salts, gives precipitates, because
the salts of these metals are but sparingly soluble in water: thus
2KX + H2SiF6 = 2HX + K2SiF6. The potassium salt is obtained in[107]
the form of very fine octahedra, but the precipitate does not form
quickly, and at first appears as a jelly. Nevertheless, the decomposition
is complete, and it is taken advantage of for obtaining their corresponding
acids from salts of potassium.[10 bis]
Silicon, having so much in common with carbon, is also able to
combine with it in the proportion given by the law of substitution,
that is, it forms a carbide of silicon CSi, called carborundum and
obtained by Mühlhäuser and Acheson in the United States, and by
Moissan in France (1891), and others, by reducing silica with carbon in
the electrical furnace at a temperature of about 2500°[11], i.e. by the
action of an electrical current upon a mixture of carbon and SiO2
with NaCl. After treating the resultant mass with acids and washing
with water, carborundum is obtained in transparent, lustrous grains
of a greenish color, possessing great hardness (greater than corundum)
and therefore used for polishing the hardest kinds of steel and stones.
The specific gravity is about 3·1. Carborundum does not alter at a
red heat, does not burn, and apparently approaches the diamond in
its properties. (Moissan obtained, 1894, a similar very hard compound
for boron, B6C, sp. gr. 2·5.)
According to the principle of substitution, if silicon forms SiH4,
a series of hydrates, or hydroxyl derivatives, ought to exist
corresponding to it. The first hydrate of an alcoholic character
ought to have the composition SiH3(OH); the second hydrate
SiH2(OH)2; the third, SiH(OH)3;[11 bis] and the last, Si(OH)4. The[108]
last is a hydrate of silica, because it is equal to SiO2 + 2H2O); and it
is formed by the action of water on silicon chloride, when all four
atoms of chlorine are replaced by four hydroxyl groups. It does not,
however, remain in this state, but easily loses part of its water.
Silica or silicic anhydride, both in the free state and in combination
with other oxides, enters into the composition of most of the rocky
formations of the earth's crust. These silicious compounds are substances
varying so much in their properties, crystalline forms, and relations
to one another that they are comprised in a special branch of
natural science (like the carbon compounds), and are treated of in
works on mineralogy; so that, in dealing with them further, we shall
only give a short description of these various compounds. It is first
of all necessary to turn to the description of silica itself, especially as
it is not unfrequently met with in nature in a separate state, and often
forms whole masses of rocky formations, called ‘quartz.’ In an anhydrous
condition silica appears in the greatest variety of natural forms—sometimes
in well-formed crystals, hexagonal prisms, terminated by
hexagonal pyramids. If the crystals are colourless and transparent,
they are called rock crystal. This is the purest form of silica. Prismatic
crystals of rock crystal sometimes attain considerable size, and
as they are remarkable for their unchangeability, great hardness, and
high index of refraction, they are used for ornaments, for seals,
making necklaces, &c.[12] Rock crystal coloured with organic matter in[109]
contact with which it has been produced has a brown or greyish colour,
and then bears the name of cairngorm or smoky quartz. In this form it
has the same uses as rock crystal, especially as it is often found in large
masses. The same mineral, frequently occurs, coloured red or pink by
manganese or iron oxides, especially in aqueous formations, and is
then known as amethyst. When finely coloured the amethyst is used
as a precious stone, but amethysts most frequently occur as small
crystals in the cavities formed in other rocky formations, and especially
in those formed in silica itself. A similar anhydrous silica
is often found in transparent non-crystalline masses, having the
same specific gravity as rock crystal itself (2·66). In this case it
is called quartz. Sometimes it forms complete rocky formations, but
more often penetrates or is interspersed through other rocky formations,
together with other siliceous compounds. Thus, in granite,
quartz is mixed with felspar and similar substances. Sometimes the
colouring of quartz is so considerable that it is hardly transparent in
thin sheets, but it is often found in transparent masses slightly coloured
with various tints. The existence in nature of enormous masses of quartz
proves that it resists the action of water. When water destroys rocky
formations, the siliceous minerals which they contain are partly dissolved
and partly transformed into clay, &c. But the quartz remains
untouched, in the form of grains in which it existed in the rocky
formation; sometimes, when crushed, it is carried away by the water
and deposited. This is the nature of sand. Naturally, sometimes
other rocky substances which are not changed by water, or only
slightly acted on by it, are found in sand; but as these latter are more
or less changed by the continuous action of water, it is not unusual to
find sand which consists almost entirely of pure quartz. Common sand
is generally coloured yellow or reddish-brown by foreign mineral matter,
consisting principally of ferruginous minerals and clays. The purest
or so-called quartz sand is, however, rarely found, and is recognised by
the absence of colour, and also by the test that when shaken in water
it does not form any turbidity: this shows the absence of clay; when
fused with bases it forms a colourless glass, and on this account is a
valuable material for the manufacture of glass. Sands were formed at
all periods of the earth's existence; the ancient ones, compressed by
strata of more recent formation and permeated with various substances
(deposited from the infiltrating water), are sometimes solidified into[110]
rock, called sandstone, composing, in some places, whole mountain
chains, and serviceable as a most excellent building material, on account
of the slight change it undergoes under the influence of atmospheric
agencies, and on account of the facility with which it may be wrought
from rocky formations into immense regularly-shaped flags—the latter
property is due to the primary laminar structure of the sand formations
deposited, as above-mentioned, by water. Many grindstones and whetstones
are made from such rocks.
Perfectly pure anhydrous silica is not only known in the condition
of rock crystal and quartz having a specific gravity of 2·6, but
also in another special form, having other chemical and physical properties.
This variety of silica has a specific gravity of 2·2, and is
formed by fusing rock crystal or heating silicic acid.[12 bis] Silicic acid,
when heated to a dull red heat, parts entirely with the water it
contains, and leaves an exceedingly fine amorphous mass of silica (easily
levigated, but difficult to moisten); it is characterised by such excessive
friability that, when lightly blown on, a large mass of it rises into the
air like a cloud of dust. A mass of anhydrous silica maybe poured in
this way from one vessel to another like a liquid, and like the latter it
takes a horizontal position in the vessel containing it.[13] Anhydrous
silica, like quartz, does not fuse in the heat of a furnace, but it fuses in
the oxyhydrogen flame to a colourless glassy mass exactly similar to that
formed in the same way from rock crystal. In this condition silica has
a specific gravity of 2·2.[13 bis] Both forms of silica are insoluble in[111]
ordinary acids, and even when they are in the state of powder,
alkalis in solution act very slowly and feebly on them; rock crystal
offers much greater resistance to the action of alkalis than the
powder obtained by heating the hydrate. The latter is quite soluble,
although but slowly, in hot alkaline solutions. This last property
appertains in a greater degree to anhydrous silica having a specific
gravity of 2·2 than to that which has a specific gravity of 2·6. Hydrofluoric
acid more easily transforms the former into silicon fluoride than
it does the latter. Both varieties of silica, when taken in the form of
powder, easily combine with bases, forming, on being fused with
an alkali, a vitreous slag, which is a salt corresponding with silica.
Glass is such a salt, formed of alkalis and alkaline earthy bases; if
the glass does not contain any of the latter—that is, if only alkaline
glass be taken—a mass soluble in water is obtained. In order to obtain
such soluble glass, potassium or sodium carbonates, or better a mixture
of the two (fusion mixture), is fused with fine sand. A still better
and further saturation of the alkalis with silica is effected by the action
of alkaline solutions on the silicon hydrate met with in nature; for
instance, an alkaline solution is often made use of to act on the so-called
tripoli, or collection of siliceous skeletons of the lowest microscopical
infusoria, which is sometimes found in considerable layers in
the form of a sandy mass. Tripoli is used for polishing, not only on
account of the considerable hardness of the silica, but also because
the microscopic bodies of the infusoria have a pointed shape, which,
however, is not angular, so that they do not scratch metals like
sand.[14] The alkaline solutions of silica obtained by boiling tripoli with
caustic soda under pressure contain various proportions of silica
and alkali.[14 bis] In order that it may contain the greatest amount of[112]
silica, silicic acid should be added to the heated solution. Silicic
acid is formed by taking any solution containing silica and alkali,
and adding to it, by degrees, some acid—for instance, sulphuric
or hydrochloric; if the experiment be carried on carefully and
the solution be concentrated, the whole mass thickens to a jelly,
due to the gelatinous form of the silicic acid separated from the
salt by the action of the acid. The decomposition may be expressed
by the following equation: Si(ONa)4 + 4HCl = 4NaCl + Si(OH)4.
The hydrate separated, Si(OH)4, easily loses part of the water and
forms a jelly, the whole mass gelatinising if the solution be strong
enough.[15]
Neither of the two varieties of anhydrous silica, nor the various
natural gelatinous hydrates, are directly soluble in water. There is,
however, a condition of silica known which is soluble in water,[113]
soluble silica, and silica is found in this state in nature. Small
quantities of soluble silica are met with in all waters. Certain mineral
springs, and especially hot springs—of which the best known are the
Geysers of Iceland and those in the North American National Park
(Yellowstone Valley)—contain a considerable amount of silica in solution.
Such water, permeating the objects it meets with—for instance,
wood—penetrates into them and deposits silica inside them, that is,
transforms them into a petrified condition. Siliceous stalactites,
and also many (if not all) forms of silica are formed by such water.
The absorption of silica by plants by means of their roots, and
also by the lower organisms having siliceous bodies, is due also to
their nourishing themselves with the solutions containing silica
continually formed in nature. Thus, in plants, in the straws of
the grasses, in hard shave-grass, and especially in the knots of
bamboo and other straw-like plants, a considerable quantity of
silica is deposited, which must previously have been absorbed by the
plants.
Silicic acid is a colloid. The gelatinous silicon hydrate is its
hydrogel, the soluble hydrate is the hydrosol (Chapter XII.) Both
varieties may be easily obtained from the alkaline silicates and from
water-glass. The very same substances—that is, aqueous solutions of
soluble glass and acid—taken in the same proportion, may produce
either the gelatinous or the soluble silica, according to the way these
solutions are mixed together. If the acid be added little by little to the
alkaline silicate, with continuous stirring, a moment arrives when the
whole mass thickens to a jelly, hydrogel; in this case the silicic acid is
formed in the midst of the alkaline solution and becomes insoluble.
But if the mixing be done in the reverse order—that is, if the soluble
glass be added to the acid, or if a quantity of acid be rapidly poured into
the solution of the salt—then the separation of the silica takes place
in the midst of the acid liquid, and it is obtained in the form of the
soluble hydrate, the hydrosol.[16]
[114]
The hydrosol of silica prepared by mixing an excess of hydrochloric
acid with a solution of sodium silicate, may be freed from the admixtures
both of hydrochloric acid and salt, sodium chloride, by means of
dialysis,[17] as Graham showed (in 1861) in enquiring into the nature of
colloids (Chapter I.), and making many other important chemical investigations.
The solution, containing the acid, salt, and silica, all
dissolved in water, is poured into a dialyser—that is, a vessel with a
porous diaphragm surrounded by water. Certain substances pass more
easily through the diaphragm than others. This may be represented
thus: the passage through the diaphragm proceeds in both directions,
and if the solutions on each side of the diaphragm be equally strong,
there will be equal numbers of molecules of the soluble substance
passing into either side in a given time, some passing quickly and
others slowly. The metallic chlorides and hydrochloric acid belong to
the series of crystalloids which easily pass through a diaphragm, and
therefore the hydrochloric acid and sodium chloride contained in the
above-mentioned dialyser pass from the solution through the diaphragm
into the water of the external vessel with considerable rapidity. The
aqueous solution of colloidal silica also penetrates through the diaphragm,
but very much more slowly. But if the amount of the substance
dissolved is not equal on either side of the diaphragm, the whole
system strives to attain a state of equilibrium; that is, the given
substance penetrates through the diaphragm from the side where it
is in excess to the part where there is a smaller quantity of it. All substances
which are soluble in water have the faculty of penetrating
through a membrane swollen in water, but the velocity of penetration
is not equal, and in this respect the dialyser separates substances like
a sieve. The silica passes less rapidly through the diaphragm than the
sodium chloride and hydrochloric acid, so that by repeatedly changing
the external water it is easy to effect the extraction of the chlorine
compounds from the dialyser, which will finally only contain a solution
of silica. This extraction (of HCl and NaCl) may be so complete that
the liquid taken from the dialyser will not give any precipitate with a
solution of silver nitrate. Graham obtained in this way soluble silica
having a distinctly acid reaction, which, however, disappeared on the
addition of a very minute quantity of alkali; for ten parts of silica in
the solution it was sufficient to take one part of alkali in order to give the[115]
liquid an alkaline reaction, so slightly energetic are the acid properties
of silicic acid. The solution of silica obtained by this method becomes
gelatinous on standing, on being heated, or on evaporation under the
receiver of an air-pump, &c. The hydrosol is transformed into the
hydrogel, the soluble hydrate into the gelatinous.
Thus in addition to the gelatinous form of the silicic acid, there
exists also a variety of this substance, soluble in water, as is the case
with alumina. Such variation in properties and exactly the same relations
with regard to water characterise an immense series of other
substances having a great significance in nature. The number of such
substances is especially great among organic compounds, and particularly
in those classes of them which compose the principal material
of the bodies of animals and plants. It is sufficient to mention, for
instance, the gelatin which is familiar to all as carpenter's and other
glues, and in the form of size and jelly. The same substance is also
known in the solution which is used to join objects together. In a
peculiar insoluble condition it enters into the composition of hides and
bones. These various forms of gelatin differ in the same way as the
different varieties of silica. The property of forming a jelly is exactly
the same as in silica, and the adhesiveness of the solutions of both substances
is identical; soluble silica adheres like a solution of gelatin.
The same properties are again shown by starch, rosin, and albumin,
and by a series of similar substances. The diaphragms used in dialysis
are also insoluble, gelatinous, forms of colloids. The bodies of
animals and plants consist largely of similar matter, insoluble in water,
corresponding with the gelatinous or insoluble silicon hydrate, or with
glue. The albumin which coagulates when eggs are boiled is a typical
form of the gelatinous condition of such substances in the body.
These slight indications are sufficient in order to show how great is
the significance of those transformations which are so well marked
in silica. The facts discovered by Graham in 1861–1864 comprise the
most essential acquisitions in the general association of these phenomena
of nature in the history of organic forms. The facility of transit
from hydrogel to hydrosol is the first condition of the possibility of
the development of organisms. The blood contains hydrosols, and the
hydrogels of the same substances are contained in the muscles and tissues,
and especially on the surface, of the body. All tissues are formed
from the blood, and in that case the hydrosols are converted into hydrogels.[18]
The absence of crystallisation, the property, apparently under
the influence of feeble agencies, of passing from the soluble condition to[116]
the insoluble, to the gelatinous condition of the hydrogel, constitute
the fundamental properties of all colloids.[19]
Silica, as regards its salt forming properties, stands in the series
of oxides on the boundary line on the side of the acids in just such a
place as alumina occupies on the side of the bases—that is, aluminium
hydroxide is the representative of the feeblest bases and silicic acid
is the least energetic of acids (at least in the presence of water—that
is, in aqueous solutions); in alumina, however, the basic properties
are distinctly expressed, while in silica the acid properties preponderate.
Like all feeble acid oxides it is capable of forming, with other acids,
saline compounds which are but slightly stable and are very easily
decomposed in the presence of water. The chief peculiarity of the silicates
consists in the number of their types. The salts formed with nitric or
sulphuric acid exist in one, two, and three tolerably stable forms, but
for acids like silicic acid the number of forms is very great, almost
unlimited. The natural silicates in particular furnish proof of this
fact; they contain various bases in combination with silica, and for
one and the same base there often exist various degrees of combination.
As feeble bases are capable of forming basic salts in addition to normal
salts—that is, a compound of a normal salt with a feeble base (either
the hydroxide or the oxide)—so the feeble acid oxides (although
not all) form, in addition to normal salts, highly acid salts—that is,
normal salts plus acid (hydrate or anhydride). Such acids are boric,
phosphoric, molybdic, chromic, and especially silicic, acid.
In order to explain these relations it is necessary first to recollect
the existence of the various hydrates of silica, or silicic acids,[20] and then[117]
to turn our attention to the similarity between silicon compounds and
metallic alloys. Silica is an oxide having the appearance of, and in
many respects the same properties as, those oxides which combine with
it, and if two metals are capable of forming homogeneous alloys in
which there exist definite or indefinite compounds, it is permissible to
assume a similar power of forming alloys in the case of analogous oxides.
Such alloys are found in indefinite, amorphous masses in the form of
glass, lava, slags, and a number of similar siliceous compounds which
do not contain any definite types of combination, but nevertheless are
homogeneous throughout their mass. By slow cooling, or under other
circumstances, definite crystalline compounds may—and sometimes do—separate
from this homogeneous mass, as also sometimes definite
crystalline alloys separate from metallic alloys.
The formation of crystalline rocks in nature is partly of such a
nature. By aqueous or igneous agency, but in any case in a liquid
condition, those oxides which form the earth's crust and her crystalline
minerals came into mutual contact. First of all they formed a shapeless
mass, of which lava, glass, slags and solutions are examples, but
little by little, or else suddenly, some definite compounds of certain
oxides existing in this alloy or in the shapeless mass were formed. This[118]
is entirely similar to two metals forming a homogeneous alloy,[21] and
under known circumstances (for instance, on cooling the alloy, or in
the case of aqueous solution when the two metals are simultaneously
liberated from the solution), definite crystalline compounds are
separated. In any case there is no doubt that there is less distinction
between silica and bases, than between bases and such anhydrides
as, for instance, sulphuric or nitric, or even carbonic, as is seen on
comparing the physical and chemical properties of silica and various
kinds of oxides. Alumina, especially, is exceedingly near akin to[119]
silica; not only in the hydrated state, but also in the anhydrous
condition, there exists a certain similarity between the crystalline forms
of alumina and silica, in the uncombined state. Both are very hard,
transparent, inactive, non-volatile, infusible, and crystallise in the
hexagonal system—in a word, they are remarkably similar, and for this
reason they are capable, like two kindred metals, of entering into many
different degrees of combination. Isomorphous mixtures—that is, differing
by the substitution of oxides akin both in their physical and chemical
characters—are very frequently met with among minerals, and the
study of the latter gave the principal impetus to the study of isomorphism.
Thus, in a whole series of minerals, lime and magnesia are
found in variable and interchangeable proportions. Exactly the same
may be said of potassium and sodium, of alumina and ferric oxide,
of manganous, ferrous, magnesium oxides, &c. Such isomorphism
does not, however, extend without change of form and properties
beyond certain rather narrow limits.[22] What I mean by this is that[120]
lime is not always replaced totally, but often only in small quantities,
by magnesia, or by the manganous and ferrous oxides, without changing
the crystalline form. The same may be observed with regard to potassium
and lithium, which may be in part, but not completely, replaced by
sodium. On the total substitution of one metal for another, often
(although not invariably) the entire nature of the substance is changed;
for instance, enstatite (or bronzite) is a magnesium bisilicate with a
small isomorphous substitution of calcium for magnesium; its composition
is expressed by the formula MgSiO3, it belongs to the rhombic
system. On the entire substitution of calcium, wollastonite, CaSiO3,
of the monoclinic system, is obtained; when manganese is substituted,
rhodonite, of the triclinic system, is produced; but in all of them the
angles of the prism are 86° to 88°.[23]
[121]
The most remarkable complex siliceous compounds are the felspars,
which enter into nearly all the primary rocks like porphyry, granite,
gneiss, &c. These felspars always contain, in addition to silica and
alumina, oxides presenting more marked basic properties, such as potash,
soda, and lime. Thus the orthoclase (adularia), or ordinary felspar
(monoclinic) of the granites, contains K2O,Al2O3,6SiO2; albite contains
the same substances, only with Na2O instead of K2O (it already appertains
to the triclinic system); anorthite contains lime, and its composition
is CaO,Al2O3,2SiO2. On expressing the two last as containing
equal quantities of oxygen, we have:—
| Albite |
Na2 |
Al2 |
Si6 |
O16 |
| Anorthite |
Ca2 |
Al4 |
Si4 |
O16 |
It is then evident that on the conversion of albite into anorthite, Na2Si2
is replaced by Ca2Al2, and this sum, both in chemical energy and in the
form of oxide, may be considered as corresponding with the first, because
sodium and silicon are extreme elements in chemical character (from
groups I. and IV.), and calcium and aluminium are means between
them (from groups II. and III.), and actually both these felspar minerals
are not only of one (triclinic) system, but form (Tchermak,
Schuster) all possible kinds of definite compounds (isomorphous
mixtures) between themselves, as indicated by their composition and
all their properties. Thus oligoclase, andesine, labradorite, &c. (plagioclases),
are nothing more than mutual combinations of albite and
anorthite. Labradorite consists of albite, in combination with 1 to 2 molecules
of anorthite. The class of zeolites corresponds to the felspars; they
are hydrated compounds of a similar composition to the felspars. Thus
natrolite contains Na2O,Al2O3,3SiO2,2H2O, and analcime presents the
same composition, but contains 4SiO2 instead of 3SiO2. In general,
the felspars and zeolites contain RO,Al2O3,nSiO2, where n varies considerably.[24]
Such complex silicates are generally insoluble in water,[25] and if[122]
[123]
they undergo change in it, it is but very slow, and more often only in
the presence of carbonic acid. Some of the silicates which are insoluble
in water are easily and directly decomposed by acids; for instance, the
zeolites and those fused silicates which contain a large quantity of
energetic bases—such as lime. Many of the silicates, like glass,[26] are[124]
hardly changed by acids, particularly if they contain much silica,
whilst fusion with alkalis leads to the formation of compounds rich in
bases, after which acids decompose the alloys formed.[27]
According to the periodic law, the nearest analogues of silicon ought
to be elements of the uneven series, because silicon, like sodium, magnesium,
and aluminium, belongs to the uneven series.[28] Immediately
after silicon follows ekasilicon or germanium, Ge = 72, whose properties
were predicted (1871) before Winkler (1886) in Freiberg, Saxony
(Chapter XV. § 5), discovered this element in a peculiar silver ore called
argyrodite, Ag6GeS5.[29] Easily reduced from the oxide by heating with[125]
hydrogen and charcoal, and separated from its solutions by zinc,
metallic germanium proved to be greyish white, easily crystallisable (in
octahedra), brittle, fusible (under a coating of fused borax) at about
900°, and easily oxidisable; the specific gravity = 5·469, the atomic
weight = 72·3, and the specific heat = 0·076,[30] as might be expected
for this element according to the periodic law. The corresponding
germanium dioxide, GeO2, is a white powder having a specific gravity
of 4·703; water, especially when boiling, dissolves this dioxide (1 part
of GeO2 requires for solution 247 parts of water at 20°, 95 parts at
100°). It forms soluble salts with alkalis and is but sparingly soluble
in acids.[31] In a stream of chlorine the metal forms germanium
chloride, GeCl4, which boils at 86°, and has a specific gravity of
1·887 at 18°; water decomposes it, forming the oxide. All these properties[32]
of germanium, showing its analogy to silicon and tin, form a
most beautiful demonstration of the truth of the periodic law.[33]
The increase of atomic weight from silicon 28 to germanium 72
is 44—that is, about the same difference as there is in the atomic
weights of chlorine and bromine; between germanium and its next
analogue, tin (Sn = 118), the difference is 46—that is, almost as much
as the amount by which the atomic weight of iodine exceeds that of
bromine.
Metallic tin is rarely met with in nature; it occurs in the veins of
ancient formations, almost exclusively in the form of oxide, SnO2, called[126]
tin-stone. The best known tin deposits are in Cornwall and in Malacca.
In Russia, tin ores have been found in small quantities on the shores of
Lake Ladoga, in Pitkarand. The crushed ore may easily be separated
from the earthy matter accompanying it by washing on inclined tables,
as the tin-stone has a specific gravity of 6·9, whilst the impurities are
much lighter. Tin oxide is very easily reduced to metallic tin by heating
with charcoal. For this reason tin was known in ancient times, and the
Phœnicians brought it from England. Metallic tin is cast into ingots of
considerable weight or into thin sticks or rods. Tin has a white colour,
rather duller than that of silver. It fuses easily at 232°, and crystallises
on cooling. Its specific gravity is 7·29. The crystalline structure of
ordinary tin is noticed in bending tin rods, when a peculiar sound is
heard, produced by the fracture of the particles of tin along the surfaces
of crystalline structure.
When pure tin is cooled to a low temperature it splits up into
separate crystals, the bond between the particles is lost, the tin assumes
a grey colour, becomes less brilliant—in a word, its properties become
changed, as Fritzsche showed. This depends on the peculiar structure
which the tin then acquires, and is particularly remarkable because
it is effected by cold in a solid.[33 bis] If such tin be fused, or even
simply heated, it becomes like ordinary tin, but is again changed when
cooled. When in this condition tin has a specific gravity of 7·19.
Similarly, tin is obtained by the action of the galvanic current on
a solution of tin chloride; it then appears in crystals of the cubic
system, and has a specific gravity of 7·18—that is, the same as when
cooled.[34]
Tin is softer than silver and gold, and is only surpassed by lead in
this respect. In addition to this it is very ductile, but its tenacity is
very slight, so that wire made from it will bear but little strain. In
consequence of its ductility it is easily worked, by forging and rolling
into very thin sheets (tin foil), which are used for wrapping many
articles to preserve them from moisture, &c. In this case, however,
and in many others, lead is mixed with the tin, which, within certain
limits, does not alter the ductility. Whilst so soft at the ordinary
temperatures tin becomes brittle at 200°, before fusing. Tin powder
may be easily obtained if the metal be fused and then stirred whilst
cooling. At a white heat tin may be distilled, but with more difficulty
than zinc. If molten tin comes into contact with oxygen, it oxidises,[127]
forming stannic oxide, SnO2, and its vapour burns with a white flame.
At ordinary temperatures tin does not oxidise, and this very important
property of tin allows it to be applied in many cases for covering other
metals to prevent their oxidising. This is termed tinning. Iron and
copper are frequently tinned. Iron and steel sheets, coated with tin,
bear the name of tin plate (for the most part made in England), and are
used for numerous purposes. Tin plate is prepared by immersing iron
sheets, previously thoroughly cleansed by acid and mechanical means,
into molten tin.[34 bis]
Tin with copper forms bronze, an alloy which is most extensively
used in the arts. Bronze has various colours and a variety of physical
properties, according to the relative amount of copper and tin
which it contains. With an excess of copper the alloy has a yellow
colour; the admixture of tin imparts considerable hardness and
elasticity to the copper. An alloy containing 78 parts of copper and
about 22 per cent. of tin is so elastic that it is used for casting bells,
which naturally require a very elastic and hard alloy.[35] For casting[128]
statues and various large or small ornamental articles alloys containing
2 to 5 p.c. of tin, 10 to 30 p.c. of zinc, and 65 to 85 p.c. of copper are
used.[36] Tin is also often used alloyed with lead, for making various
objects—for instance, drinking vessels.
Tin decomposes the vapour of water when heated with it, liberating
the hydrogen and forming stannic oxide. Sulphuric acid, diluted with a
considerable quantity of water, does not act, or at all events acts very
slightly, on tin, but tin reduces hot strong sulphuric acid, when not
only sulphurous anhydride but also sulphuretted hydrogen is evolved.
Hydrochloric acid acts very easily on tin, with evolution of hydrogen
and formation of stannous chloride, SnCl2, in solution, which, with an
excess of hydrochloric acid and access of air, is converted into stannic
chloride: SnCl2 + 2HCl + O = SnCl4 + H2O.[36 bis] Nitric acid diluted
with a considerable quantity of water dissolves tin at the ordinary temperature,
whilst the nitric acid itself is reduced, forming, amongst other
products, ammonia and hydroxylamine. Here the tin passes into solution
in the form of stannous nitrate. Stronger nitric acid (also more
dilute, when heated) transforms the tin into its highest grade of oxidation,
SnO2, but the latter then appears as the so-called metastannic[129]
acid, which does not dissolve in nitric acid, and therefore the tin does
not pass into solution. Feeble acids—for instance, carbonic and organic
acids—do not act on tin even in the presence of oxygen, because tin does
not form any powerful bases.
It is important to remark as a characteristic of tin that it is reduced
from its solutions by many metals which are more easily oxidised,
as, for instance, by zinc.
In combination, tin appears in the two types, SnX4 and SnX2,[37]
compounds of the intermediate type, Sn2X6, being also known, but these
latter pass with remarkable facility in most cases into compounds of
the higher and lower types, and therefore the form SnX3 cannot be
considered as independent.
Stannous oxide, SnO, in an anhydrous condition is obtained by
boiling solutions of stannous salts with alkalis, the first action of
the alkali being to precipitate a white hydrate of stannous oxide,
Sn(OH)2SnO. The latter when heated parts with water as easily as the
hydrate of copper oxide. In this form stannous oxide is a black crystalline
powder (specific gravity 6·7) capable of further oxidation when
heated. The hydrate is freely soluble in acids, and also in potassium
and sodium hydroxides, but not in aqueous ammonia.[38] This property
indicates the feeble basic properties of this lower oxide, which acts in
many cases as a reducing agent.[39] Among the compounds corresponding[130]
with stannous oxide the most remarkable and the one most frequently
used is stannous chloride or chloride of tin, SnCl2, also called proto-chloride
of tin (because it is the lowest chloride, containing half as
much Cl as SnCl4). It is a transparent, colourless, crystalline substance,
melting at 250° and boiling at 606°. Water dissolves it, without
visible change (in reality partial decomposition occurs, as we shall see
presently). It is also soluble in alcohol. It is obtained by heating tin
in dry hydrochloric acid gas, the hydrogen being then liberated, or
by dissolving metallic tin in hot strong hydrochloric acid and then
evaporating quickly. On cooling, crystals of the monoclinic system are
obtained having the composition SnCl2,2H2O. An aqueous solution of
this substance absorbs oxygen from the atmosphere, and gives a precipitate
containing stannic oxide. From this it follows that a solution of
stannous chloride will act as a reducing agent, a fact frequently made
use of in chemical investigations—for example, for reducing metals
from their solutions—since even mercury may be reduced to a metallic
state from its salts by means of stannous chloride. This reducing
property is also employed in the arts, especially in the dyeing industry,
where this substance in the form of a crystalline salt finds an extensive
application, and is known as tin salt or tin crystals.
Stannic oxide, SnO2, occurring in nature as tin-stone, or cassiterite, is
formed during the oxidation or combustion of heated tin in air as a
white or yellowish powder which fuses with difficulty. It is prepared
in large quantities, being used as a white vitreous mixture for coating
ordinary tiles and similar earthenware objects with a layer of
easily fusible glass or enamel. Acid solutions of stannic oxide treated
with alkalis, and alkaline solutions treated with acids, give a precipitate
of stannic hydroxide, Sn(OH)4, also known as stannic acid,
which, when heated, gives up water and leaves the anhydride, SnO2,
which is insoluble in acids, clearly showing the feebleness of its basic
character. When fused with alkali hydroxides (not with their carbonates
or acid sulphates), an alkaline compound is obtained which is
soluble in water. Stannic hydroxide, like the hydrates of silica, is a
colloidal substance, and presents several different modifications, depending
on the method of preparation, but having an identical composition;
the various hydroxides have also a different appearance, and
act differently with reagents. For instance, a distinction is made
between ordinary stannic acid and metastannic acid. Stannic acid is[131]
produced by precipitation by soda or ammonia from a freshly-prepared
solution of stannic chloride, SnCl4, in water; on drying the precipitate
thus obtained, a non-crystalline mass is formed, which is freely soluble
in strong hydrochloric or nitric acids, and also in potassium and sodium
hydroxides. This ordinary stannic acid may be still better obtained
from sodium stannate by the action of acids. Metastannic acid is
insoluble in sulphuric and nitric acids. It is obtained in the form of
a heavy white powder by treating tin with nitric acid; hydrochloric
acid does not dissolve it immediately, but changes it to such an extent
that, after pouring off the acid, water extracts the stannic chloride,
SnCl4, already formed. Dilute alkalis not only dissolve metastannic
acid, but also transform it into salts, which, slowly, yet completely,
dissolve in pure water, but are insoluble even in dilute alkali
hydroxides. Dilute hydrochloric acid, especially when boiling, changes
the ordinary hydrate into metastannic acid. On this depends, by
the way, the formation of a white precipitate, stannic hydroxide, from
solutions of stannous and stannic chlorides diluted with water. The
stannic oxide first dissolved changes under the influence of hydrochloric
acid into metastannic acid, which is insoluble in water in the
presence of hydrochloric acid. Solutions of metastannic acid differ
from solutions of ordinary stannic acid, and in the presence of alkali
they change into solutions of ordinary acid, so that metastannic acid
corresponds principally with the acid compounds of stannic oxide,
and ordinary stannic acid with the alkaline compounds.[40] Graham
obtained a soluble colloidal hydroxide; it is subject to the same transformations
that are in general peculiar to colloids.
Stannic oxide shows the properties of a slightly energetic and intermediate
oxide (like water, silica, &c.); that is to say, it forms saline
compounds both with bases and with acids, but both are easily decomposed,
and are but slightly stable. But still the acid character is
more clearly developed than the basic, as in silica, germanic oxide,[132]
and lead dioxide. This determines the character of the compounds
SnX4, corresponding to stannic chloride, SnCl4 (also called tetrachloride
of tin). It is obtained in an anhydrous condition by the direct action
of chlorine on tin, and is then easily purified, because it is a liquid
boiling at 114°, and therefore can be easily distilled. Its specific
gravity is 2·28 (at 0°), and it fumes in the open air (spiritus fumans
libavii), reacting on the moisture of the air, thus showing the
properties of a chloranhydride. Water however does not at first
decompose it, but dissolves it, and on evaporation gives the crystallo-hydrate
SnCl4,5H2O. If but little water be taken, crystals containing
SnCl4,3H2O are formed, which part with one-third of the water when
placed under the receiver of the air-pump. A large quantity of water
however, especially on heating, causes a precipitate of metastannic
acid[41] and formation of HCl.
[133]
The alkali compounds of stannic oxide—that is, the compounds in
which it plays the part of an acid, corresponding in this respect to the
compounds of silica and other anhydrides of the composition RO2—are
very easily formed and are used in the arts. Their composition in
most cases corresponds with the formula SnM2O3—that is, SnO(MO)2,
similar to CO(MO)2, where M = K, Na. Acids, even feeble acids like
carbonic, decompose the salts, like the corresponding compounds of alumina
or silica. In order to obtain potassium stannate, which crystallises
in rhombohedra, and has the composition SnK2O3,3H2O, potassium
hydroxide (8 parts) is fused, and metastannic acid (3 parts) gradually
added. Sodium stannate is prepared in practice in large quantities by
heating a solution of caustic soda with lead oxide and metallic tin.
In this last case an alkaline solution of lead oxide is formed, and the tin
acts on the solution in such a way as to reduce the lead to the metallic
state, and itself passes into solution. It is very remarkable that lead
displaces tin when in combination with acids, whilst tin, on the contrary,
displaces lead from its alkali compounds. By dissolving the
mass obtained in water, and adding alcohol, sodium stannate is precipitated,
which may then be dissolved in water and purified by
re-crystallisation. In this case it has the composition SnNa2O3,3H2O
if separated from strong solutions, and SnNa2O3,10H2O when crystallised
at a low temperature from dilute solutions. In the arts this
salt is used as a mordant in dyeing operations. With a cold solution
of sodium hydroxide metastannic acid forms a salt of the composition
(NaHO)2,5SnO2,3H2O, from which Frémy drew his conclusions concerning
the polymerism of metastannic acid. Tin, like other metals
and many metalloids, gives a peroxide form of combination or perstannic
oxide. This substance was obtained by Spring (1889) in the
form of a hydrate, H2Sn2O7 = 2(SnO3)H2O, by mixing a solution of
SnCl2, containing an excess of HCl, with freshly prepared peroxide
of barium. A cloudy liquid is then obtained, and this after being
subjected to dialysis leaves a gelatinous mass which on drying is
found to have the composition Sn2H2O7. Above 100° this substance
gives off oxygen and leaves SnO2. It is evident that SnO3 bears
the same relation to SnO2 as H2O2 to H2O or ZnO2 to ZnO, &c.
Tin occupies the same position amongst the analogues of silicon as
cadmium and indium amongst the analogues of magnesium and
aluminium respectively, and as in each of these cases the heavier
analogues with a high atomic weight and a special combination of
properties—namely, mercury and thallium—are known, so also for
silicon we have lead as the heaviest analogue (Pb = 206), with a series
of both kindred and special properties. The higher type, PbX4—for[134]
instance, PbO2—is in a chemical sense far less stable than the lower
type, PbX. The ordinary compounds of lead correspond with the
latter, and in addition to this, PbO, although not particularly energetic,
is still a decided base easily forming basic salts, PbX2(PbO)n.
Although the compounds PbX4, are unstable they offer many points of
analogy with the corresponding compounds of tin SnO2; this is seen,
for instance, in the fact that PbO2 is a feeble acid, giving the salt
PbK2O3, that PbCl4 is a liquid like SnCl4 which is not affected by
sulphuric acid, and that PbF4 gives double salts, like SnF4 or SiF4
(Brauner 1894. See Chapter II., Note 49 bis); Pb(C2H5)4 also
resembles Sn(C2H5)4 &c. All this shows that lead is a true analogue
of tin, as Hg is of cadmium.[41 bis]
Lead is found in nature in considerable masses, in the form of
galena, lead sulphide, PbS.[42] The specific gravity of galena is 7·58,
colour grey; it crystallises in the regular system, and has a fine
metallic lustre. Both the native and artificial sulphides are insoluble
in acids (hydrogen sulphide gives a black precipitate with the salts
PbX2).[42 bis] When heated, lead melts, and in the open air is either totally
or partially transformed into white lead sulphate, PbSO4, as it also is
by many oxidising agents (hydrogen peroxide, potassium nitrate).
Lead sulphate is also insoluble in water,[43] and lead is but rarely met
with in this form in nature. The chromates, vanadates, phosphates, and
similar salts of lead are also somewhat rare. The carbonate, PbCO2, is
sometimes found in large masses, especially in the Altai region. Lead
sulphide is often worked for extracting the silver which it contains;
and as the lead itself also finds manifold industrial applications, this
work is carried out on an exceedingly large scale. Many methods are
employed. Sometimes the lead sulphide is decomposed by heating it
with cast iron. The iron takes up the sulphur from the lead and forms[135]
easily-fusible iron sulphide, which does not mix with the heavier reduced
lead. But another process is more frequently used: the lead ore (it must
be clean; that is, free from earthy matter, which may be easily removed
by washing) is heated in a reverberatory furnace to a moderate temperature
with a free access of air. During this operation part of the lead
sulphide oxidises and forms lead sulphate, PbSO4, and lead oxide.
When the oxidation of part of the lead has been attained, it is necessary
to shut off the air supply and increase the temperature, then the
oxidised compounds of the lead enter into reaction with the remaining
lead sulphide, with formation of sulphurous anhydride and metallic lead.
At first from PbS + O3, PbO + SO2 are formed, and also from PbS + O4
lead sulphate PbSO4, and then PbO and PbSO4 react with the remaining
PbS, according to the equations 2PbO + PbS = 3Pb + SO2 and
also PbSO4 + PbS = 2Pb + 2SO2.[44]
The appearance of lead is well known; its specific gravity is 11·3;
the bluish colour and well-marked metallic lustre of freshly-cut lead
quickly disappear when exposed to the air, because it becomes coated
with a layer—although a very thin layer—of oxide and salts formed
by the moisture and acids in the atmosphere. It melts at 320°, and
crystallises in octahedra on cooling. Its softness is apparent from
the flexibility of lead pipes and sheets, and also from the fact that it
may be cut with a knife, and also that it leaves a grey streak when
rubbed on paper. On account of its being so soft, lead naturally
cannot be applied in many cases where most metals may be used; but
on the other hand it is a metal which is not easily changed by
chemical reagents, and as it is capable of being soldered and drawn
into sheets, &c., lead is most valuable for many technical uses. Lead
pipes are used for conveying water[45] and many other liquids, and[136]
sheet lead is used for lining all kinds of vessels containing liquids—(acids,
for instance) which act on other metals. This particularly refers
to sulphuric and hydrochloric acids, because at a low temperature they
do not act on lead, and if they form lead sulphate, PbSO4, and chloride,
PbCl2, these salts being insoluble in water and in acids, cover the
lead and protect it from further corrosion.[46] All soluble preparations
of lead are poisonous. At a white heat lead may be partially distilled;
the vapours oxidise and burn. Lead may also be easily oxidised at
low temperatures. Lead only decomposes water at a white heat,
and does not liberate hydrogen from acids, with the exception only
of very strong hydrochloric acid and then only when boiling.
Sulphuric acid diluted with water does not act on it, or only acts
very feebly at the surface; but strong sulphuric acid, when heated,
is decomposed by it, with the evolution of sulphurous anhydride. The
best solvent for lead is nitric acid, which transforms it into a soluble
salt, Pb(NO3)2.
Although acids thus have directly but little effect on lead, and
this is one of its most important practical properties, yet when air
has free access, lead (like copper) very easily reacts with many acids, even
with those which are comparatively feeble. The action of acetic acid
on lead is particularly striking and often applied in practice. If lead
be plunged into acetic acid it does not change at all and does not pass
into solution, but if part of the lead be immersed in the acid, and
the other part remain in contact with the air, or if lead be merely
covered with a thin layer of acetic acid in such a way that the air
is practically in contact with the metal, then it unites with the oxygen
of the air to form oxide, which combines with the acetic acid and
forms lead acetate, soluble in water. The formation of lead oxide is
especially marked from the fact that with a sufficient quantity of air[137]
not only is the normal lead acetate formed but also the basic
salts.[47]
When oxidising in the presence of air,[48] when heated or in the
presence of an acid at the ordinary temperature, lead forms compounds
of the type PbX2. Lead oxide, PbO, known in industry as litharge,
silberglätte (this name is due to the fact that silver is extracted from the
lead ores of this kind) and massicot. If the lead is oxidised in air at a
high temperature, the oxide which is formed fuses, and on cooling is
easily obtained in fused masses which split up into scales of a yellowish
colour, having a specific gravity of 9·3; in this form it bears the name
of litharge. Litharge is principally used for making lead salts, for
the extraction of metallic lead, and also for the preparation of
drying oils—for instance, from linseed oil.[49] When oxidised carefully[138]
and slightly heated, lead forms a powdery (not fused) oxide
known under the name of massicot. It is best prepared in the
laboratory by heating lead nitrate, or lead hydroxide. It has a
yellow colour, and differs from litharge in the greater difficulty with
which it forms lead salts with acids. Thus, for instance, when
massicot is moistened with water it does not attract the carbonic acid
of the air so easily as litharge does. It may, however, be imagined
that the cause of the difference depends only on the formation of
dioxide on the surface of the lead oxide, on which the acids do not
act. In any case lead oxide is comparatively easily soluble in nitric
and acetic acids. It is but slightly soluble in water, but communicates
an alkaline reaction to it, since it forms the hydroxide.
This hydroxide is obtained in the shape of a white precipitate by the
action of a small quantity of an alkali hydroxide on a solution of a
lead salt. An excess of alkali dissolves the hydroxide separated, which
fact demonstrates the comparatively indistinct basic properties of lead
oxide. The normal lead hydroxide, which should have the composition
Pb(OH)2, is unknown in a separate state, but it is known in combination
with lead oxide as Pb(OH)2,2PbO or Pb3O2(OH)2. The latter
is obtained in the form of brilliant, white, octahedral crystals when
basic lead acetate is mixed with ammonia and gently heated. The
basic qualities of this hydroxide are shown distinctly by its absorbing
the carbonic anhydride of the air. When an alkaline solution of the
hydroxide is boiled, it deposits lead oxide in the form of a crystalline
powder.
Lead oxide forms but few soluble salts—for instance, the nitrate
and the acetate. The majority of its salts (sulphate, PbSO4; carbonate,
PbCO3; iodide, PbI2, &c.) are insoluble in water. These salts are
colourless or light yellow if the acid be colourless. In lead oxide the
faculty of forming basic salts, PbX2nPbO or PbX2nPbH2O2, is strongly
developed. A similar property was observed in magnesium and also
in the salts of mercury, but lead oxide forms basic salts with still
greater facility, although double salts are in this case more rarely
formed.[50]
[139]
Amongst the soluble lead salts, that best known and most often
applied in practical chemistry is lead nitrate, obtained directly by
dissolving lead or its oxide in nitric acid. The normal salt, Pb(NO3)2,
crystallises in octahedra, dissolves in water, and has a specific gravity
of 4·5. When a solution of this salt acts on white lead or is boiled
with litharge, the basic salt, having a composition Pb(OH)(NO3), is
formed in crystalline needles, sparingly soluble in cold water but easily
dissolved in hot water, and therefore in many respects resembling lead
chloride. When the nitrate is heated, either lead oxide is obtained or
else the oxide in combination with peroxide.
Lead chloride, PbCl2, is precipitated from the soluble salts of lead
when a strong solution is treated with hydrochloric acid or a metallic
chloride. It is soluble in considerable quantities in hot water, and
therefore if the solutions be dilute or hot, the precipitation of lead
chloride does not occur, and if a hot solution be cooled, the salt
separates in brilliant prismatic crystals. It fuses when heated (like
silver chloride), but is insoluble in ammonia. This salt is sometimes[140]
met with in nature, and when heated in air is capable of exchanging
half its chlorine for oxygen, forming the basic salt or lead oxychloride,
PbCl2PbO, which may also be obtained by fusing PbCl2 and PbO together.
The reaction of lead chloride with water vapour leads to the same
conclusion, showing the feeble basic character of lead 2PbCl2 + H2O
= PbCl2,PbO + 2HCl. When ammonia is added to an aqueous solution
of lead chloride a white precipitate is formed, which parts with water
on being heated, and has the composition Pb(OH)Cl,PbO. This compound
is also formed by the action of metallic chlorides on other soluble
basic salts of lead.[51]
Lead carbonate, or white lead, is the most extensively used basic
lead salt. It has the valuable property of ‘covering,’ which only to a
certain extent appertains to lead sulphate and other white powdery
substances used as pigments. This faculty of ‘covering’ consists in
the fact that a small quantity of white lead mixed with oil spreads
uniformly, and if such a mixture be spread over a surface (for instance,
of wood or metal) the surface is quickly covered—that is, light does not
penetrate through even a very thin layer of superposed white lead; thus,
for example, the grain of the wood remains invisible.[52] White lead,
or basic lead carbonate, after being dried at 120°, has a composition
Pb(OH)2,2PbCO3.[53] It may be obtained by adding a solution of sodium[141]
carbonate to a solution of one of the basic salts of lead—for instance,
the basic acetate—and likewise by treating this latter with carbonic
acid. For this purpose the solution of basic acetate is poured into the
vessel f; it is prepared in the vat A, containing litharge, into which
the pump P delivers the solution of the acetate, which remains after
the action of carbonic anhydride on the basic salt. In A a basic salt is
formed having a composition approaching to Pb4(OH)6(C2H3O2)2; carbonic
anhydride, 2CO2, is passed through this solution and precipitates
white lead, Pb2(OH)2(CO3)2, and normal lead acetate, Pb(C2H3O2)2, remains
in the solution, and is pumped back into the vat A containing
lead oxide, where the normal salt is again (on being agitated) converted
into the basic salt. This is run into the vessel E, and thence into f.
Into the latter carbonic anhydride is delivered from the generator D,
and forms a precipitate of white lead.[53 bis]
Fig. 82.—Manufacture of white lead.
In order to mark the transition from lead oxide, PbO, into lead
dioxide PbO2 (plumbic anhydride), it is necessary to direct our
attention to the intermediate oxide, or red lead, Pb3O4.[54] In the arts[142]
it is used in considerable quantities, because it forms a very durable
yellowish-red paint used for colouring the resins (shellac, colophony,
&c.) composing sealing wax. It also forms a very good cheap oil
paint, used especially for painting metals, more particularly because
drying oils—for instance, hemp seed, linseed oils—very quickly dry
with red lead and with lead salts. Red lead is prepared by slightly
heating massicot, for which purpose two-storied stoves are used. In
the lower story the lead is turned into massicot, and in the higher
one, having the lower temperature (about 300°), the massicot is transformed
into red lead. Frémy and others showed the instability of red
lead prepared by various methods, and its decomposition by acids, with
formation of lead dioxide, which is insoluble in acids, and a solution
of the salts of lead oxide. The artificial production (synthesis) of red
lead by double decomposition was most important. For this purpose
Frémy mixed an alkaline solution of potassium plumbate, K2PbO3 (prepared
by dissolving the dioxide in fused potash),[54 bis] with an alkaline
solution of lead oxide. In this way a yellow precipitate of minium
hydrate is formed, which, when slightly heated, loses water and turns
into bright red anhydrous minium Pb3O4.
Minium is the first and most ordinary means of producing lead
dioxide, or plumbic anhydride, PbO2,[55] because when red lead is[143]
treated with dilute nitric acid it gives up lead oxide, and PbO2 remains,
on which dilute nitric acid does not act. The composition of minium
is Pb3O4, and therefore the action of nitric acid on it is expressed by
the equation: Pb3O4 + 4HNO3 = PbO2 + 2Pb(NO3)2 + 2H2O. The
dioxide may also be obtained by treating lead hydroxide suspended in
water with a stream of chlorine. Under these conditions the chlorine
takes up the hydrogen from the water, and the oxygen passes over to the
lead oxide.[56] When a strong solution of lead nitrate is decomposed by
the electric current, the appearance of crystalline lead dioxide is also
observed upon the positive pole; it is also found in nature in the form
of a black crystalline substance having a specific gravity of 9·4. When
artificially produced it is a fine dark powder, resisting the action of
acids, but nevertheless when treated with strong sulphuric acid it
evolves oxygen and forms lead sulphate, and with hydrochloric acid it
evolves chlorine. The oxidising property of lead dioxide depends
of course on the facility of its transition into the more stable lead
oxide, which is easily understood from the whole history of lead compounds.
In the presence of alkalis it transforms chromium oxide into
chromic acid, whilst lead chromate, PbCrO4, is formed, remaining, however,
in solution, on account of its being soluble in caustic alkalis. The
oxidising action of lead dioxide on sulphurous anhydride is most striking,
as it immediately absorbs it, with formation of lead sulphate.[144]
This is accompanied by a change of colour and development of heat,
PbO2 + SO2 = PbSO4. When triturated with sulphur the mixture
explodes, the sulphur burning at the expense of the oxygen of the lead
dioxide. Tetrachloride of lead, PbCl4, belongs to the same class of
lead compounds as PbO2. This chloride is formed by the action of
strong hydrochloric acid upon PbO2, or, in the cold, by passing a stream
of chlorine through water containing PbCl2 in suspension. The resultant
yellow solution gives off chlorine when heated. With a solution
of sal ammoniac (Nicolukin, 1885) it gives a precipitate of a double
salt, (NH4)2PbCl6 (very slightly soluble in a solution of sal ammoniac),
which when treated with strong sulphuric acid (Friedrich, 1890) gives
PbCl4 as a yellow liquid sp. gr. 3·18, which solidifies at -18°, and when
heated gives PbCl2 + Cl2. It is not acted upon by H2SO4 like SnCl4.
Tetrafluoride of lead (Brauner) belongs to the same class of compounds,
it easily forms double salts and decomposes with the evolution of
fluorine (Chapter II., Note 49 bis).[56 bis]
Amongst the elements of the second and third groups it was
observed that the elements were more basic in the even than in the
uneven series. It is sufficient to remember calcium, strontium, and
barium in the even, and magnesium, zinc, and cadmium in the uneven
series. In addition to this, in the even series, as the atomic weight
increases, in the same type of oxidation the basic properties increase
(the acid properties decrease); for example, in the second group, calcium,
strontium, barium. The same also appears in the fourth and all the
following groups. In the even series of the fourth group titanium,
zirconium, cerium, and thorium are found. All their highest oxides,
RO2, even the lightest, titanic oxide, TiO2, have more highly developed
basic properties than silica, SiO2, and in addition to this the basic
properties are more distinctly seen in zirconium dioxide, ZrO2, than
in titanic oxide, TiO2, although the acid property of combining with
bases still remains. In the heaviest oxides, cerium dioxide, CeO2, and
thorium dioxide, ThO2, no acid properties are observed, these being
both purely basic oxides. In Chapter XVII. (Note 43) we already
pointed out this higher oxide of cerium. As the above-mentioned
elements are rather rare in nature, have but little practical application,
and do not present any new forms of combination, it is unadvisable
to dwell on them in this treatise.
Titanium is found in nature in the form of its anhydride or oxide,
TiO2, mixed with silicon in many minerals, but the oxide is also found[145]
separately in the form of semi-metallic rutile (sp. gr. 4·2). Another
titanic mineral is found as a mixture in other ores, known as titanic
iron ore (in the Thuensky mountains of the southern Ural; it is known
as thuenite), FeTiO3. This is a salt of ferrous oxide and titanic anhydride.
It crystallises in the rhombohedric system, has a metallic
lustre, grey colour, sp. gr. 4·5. The third mineral in which titanium is
found in considerable quantities in nature is sphene or titanite, CaTiSiO5
= CaO,SiO2,TiO2, sp. gr. 3·5, colour yellow, green, or the like, crystallises
in tablets. The fourth, but rare, titanic mineral is peroffskite,
calcium titanate, CaTiO3; it forms blackish-grey or brown cubic
crystals, sp. gr. 4·02, and occurs in the Ural and other localities. It
may be prepared artificially by fusing sphene in an atmosphere of water
vapour and carbonic anhydride. At the end of the last century Klaproth
showed the distinction between titanic compounds and all others
then known.[57]
[146]
The comparatively rare element zirconium, Zr = 90, is very similar
to titanium, but has a more basic character. It is rarer in nature
than titanium, and is found principally in a mineral called zircon,
ZrSiO4 = ZrO2.SiO2, crystallising in square prisms, sp. gr. 4·5. It has
considerable hardness and a characteristic brownish-yellow colour, and[147]
is occasionally found in the form of transparent crystals, as a precious
stone called hyacinth.[58] Metallic zirconium was obtained, by Berzelius
and Troost, by the action of aluminium on potassium zirconofluoride in
the same way that silicon is prepared; it forms a crystalline powder,
similar in appearance to graphite and antimony, but having a very considerable
hardness, not much lustre, sp. gr. 4·15. In many respects it
resembles silicon; it does not fuse when heated, and even oxidises with
difficulty, but liberates hydrogen when fused with potash. When fused
with silica it liberates silicon. With carbon in the electrical furnace it
forms ZrC2, with hydrogen it gives ZrH2 (like CaH2, Winkler, Vol. I.,
p. 621); hydrochloric and nitric acids act feebly on it, but aqua regia[148]
easily dissolves it. It is distinguished from silicon by the fact that
hydrofluoric acid acts on it with great facility, even in the cold and
when diluted, whilst this acid does not act on silicon at all.
The very similar element thorium (Th = 232) was distinguished by
Berzelius from zirconium. It is very rarely met with, in thorite and
orangeite, ThSiO4,2H2O. The latter is isomorphous with zircon (sp. gr.
4·8).[59]
[149]
CHAPTER XIX
PHOSPHORUS AND THE OTHER ELEMENTS OF THE FIFTH GROUP
Nitrogen is the lightest and most widely distributed representative of
the elements of the fifth group, which form a higher saline oxide of the
form R2O5, and a hydrogen compound of the form RH3. Phosphorus,
arsenic, bismuth, and antimony belong to the uneven series of this
group. Phosphorus is the most widely distributed of these elements.
There is hardly any mineral substance composing the mass of the
earth's crust which does not contain some—it may be a small—amount
of phosphorus compounds in the form of the salts of phosphoric acid.
The soil and earthy substances in general usually contain from one to
ten parts of phosphoric acid in 10,000 parts. This amount, which
appears so small, has, however, a very important significance in nature.
No plant can attain its natural growth if it be planted in an artificial
soil completely free from phosphoric acid. Plants equally require the
presence of potash, magnesia, lime, and ferric oxide, among basic, and
of carbonic, sulphuric, nitric, and phosphoric anhydrides, among acid
oxides. In order to increase the fertility of a more or less poor soil,
the above-named nutritive elements are introduced into it by means of
fertilisers. Direct experiment has proved that these substances are
undoubtedly necessary to plants, but that they must be all present
simultaneously and in small quantities, and that an excess, like an
insufficiency, of one of these elements is necessarily followed by a bad
harvest, or an imperfect growth, even if all the other conditions (light,
heat, water, air) are normal. The phosphoric compounds of the soil
accumulated by plants pass into the organism of animals, in which
these substances are assimilated in many instances in large quantities.
Thus the chief component part of bones is calcium phosphate, Ca3P2O8,
and it is on this that their hardness depends.[1]
[150]
Phosphorus was first extracted by Brand in 1669, by the ignition
of evaporated urine. After the lapse of a century Scheele, who knew
of the existence of a more abundant source of phosphorus in bones,
pointed out the method which is now employed for the extraction of
this element. Calcium phosphate in bones permeates a nitrogenous
organic substance, which is called ossein, and forms a gelatin.
When bones are treated exclusively for the extraction of phosphorus,
neglecting the gelatin, they are burnt, in which case all the ossein is
burnt away. When, however, it is desired to preserve the gelatin, the
bones are immersed in cold dilute hydrochloric acid, which dissolves
the calcium phosphate and leaves the gelatin untouched; calcium
chloride and acid calcium phosphate, CaH4(PO4)2, are then obtained in
the solution. When the bones are directly burnt in an open fire their
mineral components only are left as an ash, containing about 90 per
cent. of calcium phosphate, Ca3(PO4)2, mixed with a small amount of
calcium carbonate and other salts. This mass is treated with sulphuric
acid, and then the same substance is obtained in the solution as was
obtained from the unburnt bones immersed in hydrochloric acid—i.e. the
acid calcium phosphate soluble in water, in which reaction naturally
the chief part of the sulphuric acid is converted into calcium sulphate:
Ca3(PO4)2 + 2H2SO4 = 2CaSO4 + CaH4(PO4)2.
Ca3(PO4)2 + 4HCl = 2CaCl2 + CaH4(PO4)2.
On evaporating the solution, crystallisable acid calcium phosphate
is obtained. The extraction of the phosphorus from this salt consists
in heating it with charcoal to a white heat. When heated, the
acid phosphate, CaH4(PO4)2, first parts with water, and forms the
metaphosphate, Ca(PO3)2, which for the sake of simplicity may be
regarded, like the acid salt, as composed of pyrophosphate and phosphoric
anhydride, 2Ca(PO3)2 = Ca2P2O7 + P2O5. The latter, with
charcoal, gives phosphorus and carbonic oxide, P2O5 + 5C = P2 + 5CO.[151]
So that in reality a somewhat complicated process takes place here,
yielding ultimately products according to the following equation:
2CaH4(PO4)2 + 5C = 4H2O + Ca2P2O7 + P2 + 5CO.
After the steam has come over, phosphorus and carbonic oxide distil
over from the retort and calcium pyrophosphate remains behind.[1 bis]
As phosphorus melts at about 40°, it condenses at the bottom of
the receiver in a molten liquid mass, which is cast under water in
tubes, and is sold in the form of sticks. This is common or yellow
phosphorus. It is a transparent, yellowish, waxy substance, which is
not brittle, almost insoluble in water, and easily undergoes change in
its external appearance and properties under the action of light, heat,
and of various substances. It crystallises (by sublimation or from its
solution in carbon bisulphide) in the regular system, and[2] (in contradistinction
to the other varieties) is easily soluble in carbon bisulphide,
and also partially in other oily liquids. In this it recalls common[152]
sulphur. Its specific gravity is 1·84. It fuses at 44°, and passes into
vapour at 290°; it is easily inflammable, and must therefore be
handled with great caution; careless rubbing is enough to cause phosphorus
to ignite. Its application in the manufacture of matches is
based on this.[2 bis] It emits light in the air owing to its slow[3] oxidation,
and is therefore kept under water (such water is phosphorescent
in the dark, like phosphorus itself). It is also very easily oxidised by
various oxidising agents and takes up the oxygen from many substances.[3 bis]
Phosphorus enters into direct combination with many
metals and with sulphur, chlorine, &c., with development of a considerable
amount of heat. It is very poisonous although not soluble in
water.
Besides this, there is a red variety of phosphorus, which differs considerably
from the above. Red phosphorus (sometimes wrongly called
amorphous phosphorus) is partially formed when ordinary phosphorus[153]
remains exposed to the action of light for a long time. It is also
formed in many reactions; for example, when ordinary phosphorus
combines with chlorine, bromine, iodine, or oxygen, a portion of it is
converted into red phosphorus. Schrötter, in Vienna, investigated this
variety of phosphorus, and pointed out by what methods it may be
produced in considerable quantities. Red phosphorus is a powdery red-brown
opaque substance of specific gravity 2·14. It does not combine
so energetically with oxygen and other substances as yellow phosphorus,
and evolves less heat in combining with them.[4] Common phosphorus
easily oxidises in the air; red phosphorus does not oxidise at all at the
ordinary temperature; hence it does not phosphoresce in the air, and
may be very conveniently kept in the form of powder. It does not, like
yellow phosphorus, fuse at 44°. After being converted into vapour at[154]
290° or 300°, it again passes into the ordinary variety when slowly
cooled. Red phosphorus is not soluble in carbon bisulphide and other
oily liquids, which permits of its being freed from any admixture of the
ordinary phosphorus. It is not poisonous, and is used in many cases for
which the ordinary phosphorus is unsuitable or dangerous; for example,
in the manufacture of matches, which are then not poisonous or inflammable
by accidental friction, and therefore the red variety has now
replaced the ordinary phosphorus.[4 bis]
The heads of the ‘safety’ matches do not contain any phosphorus,
but only substances capable of burning and of supporting combustion.
Red phosphorus is spread over a surface on the box, and it
is the friction against this phosphorus which ignites the matches.
There is no danger of the matches taking fire accidentally, nor are
they poisonous.[5] This red phosphorus is prepared by heating the
ordinary phosphorus at 230° to 270°; it is evident that this must
be done in an atmosphere incapable of supporting combustion—for
example, in nitrogen, carbonic anhydride, steam, &c. On a[155]
large scale, ordinary phosphorus is placed in closed iron vessels,[5 bis] and
immersed in a bath of different proportions of tin and lead, by which
means the temperature of 250° necessary for the conversion is easily
attained. It is kept at this temperature for some time. The temperature
is at first cautiously raised, and the air is thus partially expelled
by the heat, and also by the evolution of steam (the phosphorus is
damp when put in), whilst the remaining oxygen is also partially
absorbed by the phosphorus, so that an atmosphere of nitrogen is
produced in the iron vessel. Red phosphorus enters into all the
reactions proper to yellow phosphorus, only with greater difficulty
and more slowly;[6] and, as its vapour tension (volatility) is less than[156]
that of the yellow variety, it may be supposed that a polymerisation
takes place in the passage of the yellow into the red modification, just as
in the passage of cyanogen into paracyanogen, or of cyanic acid into
cyanuric acid (Chapter IX. Notes 39 bis and 48).
The vapour of phosphorus is colourless; its density remains constant
between 300° and 1000° (Dumas, 1833; Mitscherlich, Deville, and
Troost, 1859, and others). The density with respect to air has been
determined as from 4·3 to 4·5. Hence, referred to hydrogen, it is
4·4 × 14·4 = 63, corresponding with a molecular weight 124, i.e. the
molecule of phosphorus in a state of vapour contains P4. The reader
will remember that the molecule of nitrogen contains N2, of sulphur S6
or S2, and of oxygen O2 or O3.
The chemical energy of phosphorus in a free state more nearly
approaches that of sulphur than nitrogen. Phosphorus is combustible and
inflames at 60°; but having in the act of combination parted with a portion
of its energy in the form of heat it becomes analogous to nitrogen,
so long as there is no question of its reduction back again into phosphorus.
Nitric acid is easily reduced to nitrogen, whilst phosphoric
acid is reduced with very much greater difficulty. All the compounds of
phosphorus are less volatile than those of nitrogen. Nitric acid, HNO3,
is easily distilled; metaphosphoric acid, HPO3, is generally said to be[157]
non-volatile; triethylamine, N(C2H5)3, boils at 90°, and triethylphosphine,
P(C2H5)3, at 127°.
Phosphorus not only combines easily and directly with oxygen, but
also with chlorine, bromine, iodine, sulphur, and with certain metals,
and red phosphorus when heated combines with hydrogen also.[6 bis] So, for
instance, when fused with sodium under naphtha, phosphorus gives the
compound Na3P2. Zinc, absorbing the vapour of phosphorus, gives the
phosphide Zn3P2 (sp. gr. 4·76); tin, SnP; copper, Cu2P; even platinum
combines with phosphorus (PtP2, sp. gr. 8·77).[6 tri] Iron, when combined
even with a small quantity of phosphorus, becomes brittle.[7] Some of
these compounds of phosphorus are obtained by the action of phosphorus
on the solutions of metallic salts, and by the ignition of metallic
oxides in the vapour of phosphorus, or by heating mixtures of phosphates
with charcoal and metals. Phosphides do not exhibit the
external properties of salts, which are so clearly seen in the chlorides
and still distinctly observable in the sulphides. The phosphides of the
metals of the alkalis and of the alkaline earths are even immediately
and very easily decomposed by water, whereas this is found to be the
case with only a very few sulphides, and still more rarely and indistinctly
with the chlorides. We may take calcium phosphide as an example.[7 bis][158]
Phosphorus is laid in a deep crucible, and covered with a clay plug,
over which lime is strewn. At a red heat the vapours of phosphorus
combine with the oxygen of the lime and form phosphoric anhydride,
which forms a salt with another portion of the lime, whilst the
liberated calcium combines with the phosphorus and forms calcium
phosphide. Its composition is not quite certain; it may be CaP
(corresponding with liquid phosphuretted hydrogen). This substance
is remarkable for the following reaction: if we take water—or, better
still, a dilute solution of hydrochloric acid—and throw calcium phosphide
into it, bubbles of gas are evolved, which take fire spontaneously
in the air and form white rings. This is owing to the fact
that the liquid hydrogen phosphide, PH2, is first formed, thus,
CaP + 2HCl = CaCl2 + PH2, which, owing to its instability, very
easily splits up into the solid phosphide, P2H, and gaseous phosphide,
PH3; 5PH2 = P2H + 3PH3; the latter corresponds with ammonia.
The mixture of the gaseous and liquid phosphides takes fire spontaneously
in the air, forming phosphoric acid. The same hydrogen
phosphides are formed when water acts on sodium phosphide (P2Na3).
A similar mixture of gaseous liquid and solid phosphuretted hydrogen
(Retgers 1894) is formed by heating (in a glass tube) red phosphorus
in a stream of dry hydrogen. Hence we see that there are three compounds
of phosphorus with hydrogen. (1) The first or solid yellow
phosphide, P2H (more probably P4H2), is obtained by the action of
strong hydrochloric acid on sodium phosphide; it takes fire when
struck or at 175°. (2) The liquid, PH2, or more correctly expressed as
the molecule, P2H4, is a colourless liquid which takes fire spontaneously
in the air, boils at 30°, is very unstable, and is easily decomposed (by
light or hydrochloric acid) into the two other phosphides of hydrogen.
It is prepared by passing the gases evolved by the action of water
on calcium phosphide through a freezing mixture.[8] And, lastly, (3),
gaseous hydrogen phosphide, phosphine, PH3, which is distinguished as
being the most stable. It is a colourless gas, which does not take fire
in the air. It has an odour of garlic, and is very poisonous. It[159]
resembles ammonia in many of its properties.[8 bis] It is easily decomposed
by heat, like ammonia, forming phosphorus and hydrogen; but
it is very slightly soluble in water, and does not saturate acids,
although it forms compounds with some of them which resemble
ammonium salts in their form and properties. Among them the compound
with hydriodic acid, PH4I, analogous to ammonium iodide, is
remarkable. This compound crystallises on sublimation in well-formed
cubes, like sal-ammoniac, which it resembles in many respects. However,
this compound does not enter into those reactions of double
decomposition which are proper to sal-ammoniac, because its saline
properties are very feebly developed. Phosphuretted hydrogen also
combines, like ammonia, with certain chloranhydrides; but they are
decomposed by water, with the evolution of phosphine. Ogier (1880)
showed that hydrochloric acid also combines with phosphine under a
pressure of 20 atmospheres at +18°, and under the ordinary pressure
at -35°, forming the crystalline phosphonium chloride PH4Cl, corresponding
to sal-ammoniac. Hydrobromic acid does the same with greater
ease, and hydriodic acid with still greater facility, forming phosphonium
iodide, PH4I.[9]
[160]
Phosphuretted hydrogen, or phosphine, PH3, is generally prepared by
the action of caustic potash on phosphorus.[10] Small pieces of phosphorus
are dropped into a flask containing a strong solution of caustic
potash and heated. Potassium hypophosphite, H2KPO2, is then obtained
in solution; gaseous phosphuretted hydrogen is evolved:
P4 + 3KHO + 3H2O = 3(KH2PO2) + PH3.
Liquid phosphuretted hydrogen (and free hydrogen) is also formed,
together with the phosphine, so that the gaseous product, on escaping
from the water into the air, takes fire spontaneously, forming beautiful
white rings of phosphoric acid. In this experiment, as in that with
calcium phosphide, it is the liquid, P2H4, that takes fire; but the
phosphine set light to by it also burns, PH3 + O4 = PH3O4. The same
phosphuretted hydrogen, PH3, may be obtained pure, and not spontaneously
combustible, by igniting the hydrates of phosphorous acid
(4PH3O3 = PH3 + 3PH3O4) and hypophosphorous acid (2PH3O2
= PH3 + PH3O4); or, more simply, by the decomposition of calcium
phosphide by hydrochloric acid, because then all the liquid phosphide,
P2H4, is decomposed into non-volatile P2H and gaseous PH3. Pure
phosphine liquefies when cooled to -90°, boils at -85°, and solidifies
at -135° (Olszewski). When phosphorus burns in an excess[10 bis] of dry[161]
oxygen, then only phosphoric anhydride, P2O5 is formed. It is
prepared by dropping pieces of phosphorus through a wide tube, fixed
into the upper neck of a large glass globe, on to a cup suspended in the
centre of the globe. These lumps are set alight by touching them with
a hot wire, and the phosphorus burns into P2O5. The dry air necessary
for its combustion is forced into the globe through a lateral neck, and
the white flakes of phosphoric anhydride formed are carried by the
current of air through a second lateral neck into a series of Woulfe's
bottles, where they settle as friable white flakes. Phosphoric anhydride
may also be formed by passing dry air through a solution of phosphorus
in carbon bisulphide. All the materials for the preparation of this substance
must be carefully dried, because it combines with great eagerness
with water, at the same time developing a large amount of heat and
forming metaphosphoric acid, HPO3, from which the water cannot
be separated by heat. Phosphoric anhydride is a colourless snow-like
substance, which attracts moisture from the air with the utmost avidity.
It fuses at a red heat, and then volatilises. Its affinity for water is so
great that it takes it up from many substances. Thus it converts sulphuric
acid into sulphuric anhydride, and carbohydrates (wood, paper)
are carbonised, and give up the elements of water when brought into
contact with it.
When moist phosphorus slowly oxidises in the air, it not only
forms phosphorous and phosphoric acids, but also hypophosphoric acid,
H4P2O6, which when in a dry state easily splits up at 60° into phosphorous
and metaphosphoric acids (H4P2O6 = H3PO3 + HPO3), but
differs from a mixture of these acids in that it forms well-characterised
salts, of which the sodium salt, H2Na2P2O6, is but slightly soluble in
water (the sodium salts of phosphoric and phosphorous acids are easily
soluble), and that it does not act as a reducing agent, like mixtures
containing phosphorous acid.[11]
[162]
Judging by the general law of the formation of acids (Chapter XV.),
the series of phosphorus compounds should include the following ortho-acids
and their corresponding anhydrides, answering to phosphuretted
hydrogen, H3P:—
| H3PO4, |
phosphoric acid, and |
P2O5, |
anhydride, |
| H3PO3, |
phosphorous acid, and |
P2O3, |
anhydride, |
| H3PO2, |
hypophosphorous acid, and |
P2O, |
anhydride.[12] |
The last of these (the analogue of N2O) is almost unknown. Phosphoric
anhydride (P2O5) with a small quantity of water does not at first
give orthophosphoric acid, PH3O4, but a compound P2O5,H2O, or
PHO3, whose composition corresponds with that of nitric acid; this is
metaphosphoric acid. Even with an excess of water, combining with
phosphoric anhydride, this metaphosphoric acid, and not the ortho-,
passes at first into solution. Metaphosphoric acid in solution only
passes into orthophosphoric acid when the solution is heated or after
a lapse of time.
Orthophosphoric acid[13] is obtained by oxidising phosphorus with
nitric acid until the phosphorus entirely passes into solution and the[163]
lower oxides of nitrogen cease to be evolved. The reaction takes place
best with dilute nitric acid, and when aided by heat. The resultant
solution is evaporated to a syrup. If a weighed quantity of phosphorus
(dried in a current of dry carbonic anhydride) be taken, a crystalline
mass of the acid can be obtained by evaporating the solution until it consists
only of the quantity[14] of phosphoric acid corresponding with the
amount of phosphorus taken (from 31 parts of P, 98 parts of solution).
The acid fuses at +39°; specific gravity of the liquid 1·88. Phosphorus
pentachloride, PCl5, and oxychloride, POCl3 (see further on), give
orthophosphoric acid and hydrochloric acid with water. The two other
varieties of phosphoric acid, with which we shall presently become
acquainted, give the same ortho-acid when under the influence of acids,
with particular ease when boiled and more slowly in the cold. By
itself orthophosphoric acid (either in solution or when dry) does not
pass into the other varieties; it does not oxidise, and therefore presents
the limiting and stable form. When heated to 300°, it loses water and
passes into pyrophosphoric acid, 2H3PO4 = H2O + H4P2O7, whilst at a
red heat it loses twice as much water and is converted into metaphosphoric
acid, H3PO4 = H2O + HPO3. In aqueous solution orthophosphoric
acid differs clearly from pyro- or metaphosphoric acids, because
the solutions of these latter acids give different reactions: thus orthophosphoric
acid does not precipitate albumin, does not give a precipitate
with barium chloride, and forms a yellow precipitate of silver orthophosphate,
Ag3PO4, with silver nitrate (in the presence of alkalis, but
not otherwise); whilst a solution of pyrophosphoric acid, H4P2O7,
although it does not precipitate albumin or barium chloride, gives
a white precipitate of silver pyrophosphate, Ag4P2O7, with silver
nitrate; and a solution of metaphosphoric acid, HPO3, precipitates
both albumin and barium chloride, and gives a white precipitate of
silver metaphosphate, AgPO3, with silver nitrate. These points of
distinction were studied by Graham, and are exceedingly instructive.
They show that the solution of a substance does not determine
the maximum of chemical combination with water, that solutions may
contain various degrees of combination with water, and that there is
a clear difference between the water serving for solution and that
entering into chemical combination. Graham's experiments also
showed that the water whose removal or combination determines the[164]
conversion of ortho- into meta- and pyrophosphoric acids differs distinctly
from water of crystallisation, for he obtained the salts of ortho-,
meta-, and pyrophosphoric acids with water of crystallisation, and they
differed in their reactions, like the acids themselves. This water of
crystallisation was expelled with greater ease than the water of constitution
of the hydrates in question.[14 bis]
Orthophosphoric acid has a pleasant acid taste and a distinctly acid
reaction; it is used as a medicine, and is not poisonous (phosphorous
acid is poisonous). Alkalis, like sodium, potassium, and ammonium
hydroxides, saturate the acid properties of phosphoric acid when taken
in the ratio 2NaHO : H3PO4—that is, when salts of the composition
HNa2PO4 are formed. When taken in the ratio NaHO : H3PO4, a
solution having an acid reaction is obtained, and when 3NaHO : H3PO4—that
is, when the salt Na3PO4 is formed—an alkaline reaction is
obtained. Hence many chemists (Berzelius) even regarded the salts
of composition R2HPO4 as normal, and considered phosphoric acid
to be bibasic. But the salt Na2HPO4 also shows a feeble alkaline
reaction, so that it is impossible to judge the characteristic peculiarities
of acids by the reactions on litmus paper, as we already know from
many examples. Orthophosphoric acid is tribasic, because it contains
three equivalents of hydrogen replaceable by metals, forming salts,
such as NaH2PO4, Na2HPO4, and Na3PO4. It is also tribasic, because
with silver nitrate its soluble salts always give Ag3PO4,[15] a salt with
three equivalents of silver, and because by double decomposition with[165]
barium chloride it forms a salt of the composition Ba3(PO4)2, and
silver and barium hardly ever give basic salts. With the metals
of the alkalis, phosphoric acid forms soluble salts, but the normal
salts of the metals of the alkaline earths, R3(PO4)2 and even
R2H2(PO4), are insoluble in water, but dissolve in feeble acids, such
as phosphoric and acetic, because they then form soluble acid salts,
especially RH4(PO4)2.[16]
[166]
Phosphoric anhydride, or any of its hydrates, when ignited with an
excess of sodium hydroxide, carbonate, &c., forms normal or trisodium
orthophosphate, Na3PO4, but when a solution of sodium carbonate is
decomposed by orthophosphoric acid, only the salt Na2HPO4 is
formed; and when an excess of sodium chloride is ignited with
orthophosphoric acid, hydrochloric acid is evolved, and the acid salt
H2NaPO4 alone is formed. These facts clearly indicate the small
energy of phosphoric acid with respect to the formation of the tri-metallic
salt, which is seen further from the fact that the salt Na3PO4
has an alkaline reaction, decomposes in the presence of water and carbonic
acid, forming Na2HPO4, corrodes glass vessels in which it is
boiled or evaporated, just like solutions of the alkalis, disengages, like
them, ammonia from ammonium chloride, and crystallises from solutions,
as Na3PO4,12H2O, only in the presence of an excess of alkali.
At 15° the crystals of this salt require five parts of water for solution;
they fuse at 77°.
Disodium orthophosphate, or common sodium phosphate, Na2HPO4,
is more stable both in solution and in the solid state. As it is used in
medicine and in dyeing, it is prepared in considerable quantities, most
frequently from the impure phosphoric acid obtained by the action of
sulphuric acid on bone ash. The solution thus formed—which contains,
besides phosphoric and sulphuric acids, salts of sodium, calcium, and
magnesium—is heated, and sodium carbonate added so long as carbonic
anhydride is disengaged. A precipitate is formed containing the
insoluble salts of magnesium and calcium, whilst the solution contains
sodium phosphate, Na2HPO4, with a small quantity of other salts, from
which it may be easily purified by crystallisation. At the ordinary
temperature its solutions, especially in the presence of a small amount
of sodium carbonate, give finely-formed inclined prismatic crystals,
Na2HPO4,12H2O; when the crystallisation takes place above 30°
they only contain 7H2O. The former crystals even lose a portion of
their water of crystallisation at the ordinary temperature (the salt
effloresces), and form the second salt with 7H2O; whilst under the
receiver of an air-pump and over sulphuric acid they also part with
this water.[17] When ignited they lose the last molecule of water of
constitution, and give sodium pyrophosphate, Na4P2O7.
[167]
Monosodium orthophosphate, NaH2PO4, crystallises with one
equivalent of water; its solution has an acid reaction. At 100° the
salt only loses this water of crystallisation, and at about 200° it parts
with all its water, forming the metaphosphate NaPO3. It is prepared
from ordinary sodium phosphate by adding phosphoric acid
until the solution does not give a precipitate with barium chloride, and
then evaporating and crystallising the solution. The solution of this
salt does not absorb carbonic anhydride, and does not give a precipitate
with salts of calcium, barium, &c.[18]
[168]
As a hydrate, orthophosphoric acid should be expressed, after the
fashion of other hydrates, as containing three water residues (hydroxyl
groups), i.e. as PO(OH)3. This method of expression indicates that
the type PX5, seen in PH4I, is here preserved, with the substitution
of X2 by oxygen and X3 by three hydroxyl groups. The same
type appears in POCl3, PCl5, PF5, &c. And if we recognise phosphoric
acid as PO(OH)3, we should expect to find three anhydrides
corresponding with it: (1) [PO(OH)2]2O, in which two of the three
hydroxyls are preserved; this is pyrophosphoric acid, H4P2O7. (2)
PO(OH)O, where only one hydroxyl is preserved. This is metaphosphoric
acid. (3) (PO)2O3 or P2O5, that is, perfect phosphoric anhydride.
Therefore, pyro- and metaphosphoric acids are imperfect anhydrides (or
anhydro-acids) of orthophosphoric acid.[19]
[169]
Pyrophosphoric acid, H4P2O7, is formed by heating orthophosphoric
acid to 250° when it loses water.[19 bis] Its normal salts are
formed by igniting the dimetallic salts of orthophosphoric acid of
the types HM2PO4. Thus from the disodium salt we obtain sodium
pyrophosphate, Na4P2O7 (it crystallises from water with 10H2O, is
very stable, fuses when heated, has an alkaline reaction, and does not
form ortho-salts when its solution is boiled): and from the monosodium
salt NaH2PO4 the acid salt Na2H2P2O7 (easily soluble in
water) is formed; this has an acid reaction, and when ignited further
gives the meta-salt.[20]
Metaphosphoric acid, HPO3 (the analogue of nitric acid), is formed
by the ignition of the pyro- and ortho-acids (or, better, of their
ammonium salts), as a vitreous, hygroscopic, fused mass (glacial phosphoric
acid, acidum phosphoricum glaciale), soluble in water and
volatilising without decomposition. It is also formed in the first
slow action of cold water on the anhydride, but metaphosphoric acid
gradually changes into the ortho-acid when its solution is boiled, or
when it is kept for any length of time, especially in the presence of
acids.[21]
[170]
[171]
In order to see the relation between phosphoric acid and the lower
acids of phosphorus, it is simplest to imagine the substitution of
hydroxyl in H3PO4 or PO(OH)3 by hydrogen. Then from orthophosphoric
acid, PO(OH)3, we shall obtain phosphorous acid, POH(OH)2,
and hypophosphorous acid, POH(OH); and, furthermore, phosphorous
acid should be bibasic if orthophosphoric acid was tribasic, and
hypophosphorous acid should be monobasic. This conclusion[21 bis] is,
in fact, true, and hence all the acids of phosphorus may be referred
to one common type, PX5, whose representatives are PH4I and
PCl5, POCl3, PCl2F3, &c.
Phosphorous acid, PH3O3, is generally obtained from phosphorus
trichloride, PCl3, by the action of water: PCl3 + 3H2O = 3HCl
+ PH3O3. Both acids formed are soluble in water, but are easily
separated, because hydrochloric acid is volatile whilst phosphorous
acid volatilises with difficulty, and if a small amount of water be
originally taken the hydrochloric acid nearly all passes off directly.
Concentrated solutions of phosphorous acid give crystals of H3PO3,
which fuse at 70°, attract moisture from the air, and deliquesce when
ignited, giving phosphine and phosphoric acid,[22] and are oxidised into[172]
orthophosphoric acid by many oxidising agents. In its salts only two
hydrogen atoms are replaced by metals (Würtz); the salts of the
alkaline metals are soluble, and give precipitates with salts of the
majority of other metals.
The monobasic hypophosphorous acid, PH3O2, gives salts PH2O2Na,
(PH2O2)2Ba, &c.; the two remaining atoms of hydrogen (which
exist in the same form as in phosphine, PH3) are not replaceable by
metals, and this determines the property of these salts of evolving
phosphuretted hydrogen when heated (especially with alkalis). In
acting on substances liable to reduction it is this hydrogen which acts,
and, for example, reduces gold and mercury from the solutions of
their salts, or converts cupric into cuprous salts. In all these instances
the hypophosphorous acid is converted into phosphoric acid. Under
the action of zinc and sulphuric acid it gives phosphine, PH3. Nevertheless,
neither hypophosphorous acid nor its dry salts absorb oxygen
from the air. The salts of hypophosphorous acid are more soluble
than those of the preceding acids of phosphorus. Thus the sodium
salt PNaH2O2 does not give a precipitate with barium chloride, and
the salts of calcium, barium, and many other metals are soluble.[23]
The hypophosphites are prepared by boiling an alkali with phosphorus
so long as phosphuretted hydrogen is evolved. The acid itself is
obtained from barium hypophosphite (prepared in the same manner by
boiling phosphorus in baryta water), by decomposing its solution with
sulphuric acid. By concentration of the solution of hypophosphorous
acid (it must not be heated above 130°, at which temperature it
decomposes) a syrup is formed which is able to crystallise. In the
solid state hypophosphorous acid fuses at +17°, and has the properties
of a clearly defined acid.
The types PX3 and PX5, which are evident for the hydrogen and
oxygen compounds of phosphorus, are most clearly seen in its halogen
compounds,[24] to the consideration of which we will proceed, fixing[173]
our attention more especially on the chlorine compounds, as being
the most important from the historical, theoretical, and practical point
of view.
Phosphorus burns in chlorine, forming phosphorous chloride, PCl3,
and with an excess of chlorine, phosphoric chloride, PCl5. The
oxychloride, POCl3, as the simplest chloranhydride according to the
type PX5, and also phosphoric chloride, correspond with orthophosphoric
acid, PO(OH)3, while phosphorous chloride, PCl3, corresponds
with phosphorous acid and the type PX3. Phosphoric oxychloride,
POCl3, is a colourless liquid, boiling at 110°. Phosphorus trichloride
is also a colourless liquid, boiling at 76°,[25] whilst phosphoric chloride[174]
is a solid yellowish substance, which volatilises without melting at
about 168°. They are all heavier than water, and form types of
the chloranhydrides or chlorine compounds of the non-metallic elements
whose hydrates are acids, just as NaCl or BaCl2 are types of halogen
metallic salts.
If a piece of phosphorus be dropped into a flask containing chlorine,
it burns when touched with a red-hot wire, and combines with the
chlorine. If the phosphorus be in excess, liquid phosphorus trichloride,
PCl3, is always formed, but if the chlorine be in excess the
solid pentachloride is obtained. The trichloride is generally prepared
in the following manner. Dry chlorine (passed through a series
of Woulfe's bottles containing sulphuric acid) is led into a retort containing
sand and phosphorus. The retort is heated, the phosphorus
melts, spreads through the sand, and gradually forms the trichloride,
which distils over into a receiver, where it condenses.
Phosphoric chloride or phosphorus pentachloride, PCl5, is prepared by
passing dry chlorine into a vessel containing phosphorus trichloride
(purified by distillation). Phosphorous chloride combines directly with
oxygen, but more rapidly with ozone or with the oxygen of potassium
chlorate (3PCl3 + KClO3 = 3POCl3 + KCl), forming phosphorus oxychloride,
POCl3 (Brodie). This compound is also formed by the first
action of water on phosphoric chloride; for example, if two vessels,
one containing phosphoric chloride and the other water, are placed
under a bell jar, after a certain time the crystals of the chloride
disappear and hydrochloric acid passes into the water. The aqueous
vapour acts on the pentachloride, and the following reaction occurs:
PCl5 + H2O = POCl3 + 2HCl, the result being that liquid phosphorus[175]
oxychloride is found in one vessel, and a solution of hydrochloric acid in
the other. However, an excess of water directly transforms phosphoric
chloride into orthophosphoric acid, PCl5 + 4H2O = PH3O4 + 5HCl,[26]
since POCl3 reacts with water (3H2O), forming 3HCl and phosphoric
acid PO(OH)3.
The above chlorine compounds serve not only as a type of the
chloranhydrides, but also as a means for the preparation of other
acid chloranhydrides. Thus the conversion of acids XHO into chloranhydrides,
XCl, is generally accomplished by means of phosphorus
pentachloride. This fact was discovered by Chancel, and adopted by
Gerhardt as an important method for studying organic acids. By this
means organic acids, containing, as we know, RCOOH (where R is a
hydrocarbon group, and where carboxyl may repeat itself several times
by replacing the hydrogen of hydrocarbon compounds), are converted
into their chloranhydrides, RCOCl. With water they again form the
acid, and resemble the chloranhydrides of mineral acids in their
general properties.
Since carbonic acid, CO(OH)2, contains two hydroxyl groups, its
perfect chloranhydride, COCl2, carbonic oxychloride, carbonyl chloride
or phosgene gas, contains two atoms of chlorine, and differs from
the chloranhydrides of organic acids in that in them one atom of
chlorine is replaced by the hydrocarbon radicle RCOCl, if R be a
monatomic radicle giving a hydrocarbon RH. It is evident, on the
one hand, that in RCOCl the hydrogen is replaced by the radicle
COCl, which is also able to replace several atoms of hydrogen (for
example, C2H4(COCl)2 corresponds with the bibasic succinic acid);
and, on the other hand, that the reactions of the chloranhydrides of[176]
organic acids will answer to the reactions of carbonyl chloride, as the reactions
of the acids themselves answer to those of carbonic acid. Carbonyl
chloride is obtained directly from dry carbon monoxide and chlorine[27]
exposed to the action of light, and forms a colourless gas, which easily
condenses into a liquid, boiling at +8°, specific gravity 1·43, and having
the suffocating odour belonging to all chloranhydrides. Like all chloranhydrides,
it is immediately decomposed by water, forming carbonic
anhydride, according to the equation COCl2 + H2O = CO2 + 2HCl,
and thus expresses the type proper to all chloranhydrides of both
mineral and organic acids.[28]
In order to show the general method for the preparation of acid
chloranhydrides, we will take that of acetic acid, CH3·COOH, as an example.
Phosphorus pentachloride is placed in a glass retort, and acetic
acid poured over it; hydrochloric acid is then evolved, and the substance
distilling over directly after is a very volatile liquid, boiling at 50°,
and having all the properties of the chloranhydrides. With water it[177]
forms hydrochloric and acetic acids. The reaction here taking place
may be explained thus: the substitution of the oxygen taken from the
acetic acid (from its carboxyl) by two atoms of chlorine from the
PCl5 should be as follows: CH3·COOH + PCl5 = CH3·COHCl2 + POCl3.
But the compound CH3·COHCl2 does not exist in a free state (because
it would indicate the possibility of the formation of compounds of
the type CX6, and carbon only gives those of the type CX4); it
therefore splits up into HCl and the chloranhydride CH3·COCl. The
general scheme for the reaction of phosphorus pentachloride with
hydrates ROH is exactly the same as with water; namely, ROH with
PCl5, gives POCl3 + HCl + RCl—that is a chloranhydride.[28 bis]
Containing, as they do, chlorine, which easily reacts with hydrogen,
phosphorus pentachloride, trichloride, and oxychloride enter into
reaction with ammonia, and give a series of amide and nitrile compounds[178]
of phosphorus. Thus, for example, when ammonia acts on the
oxychloride we obtain sal-ammoniac (which is afterwards removed by
water) and an orthophosphoric triamide, PO(NH2)3, as a white insoluble
powder on which dilute acids and alkalis do not act, but which,
when fused with potassium hydroxide, gives potassium phosphate and
ammonia like other amides. When ignited, the triamide liberates
ammonia and forms the nitrile PON, just as urea, CO(NH2)2,
gives off ammonia and forms the nitrile CONH. This nitrile, called
monophosphamide, PON, naturally corresponds with metaphosphoric
acid, namely, with its ammonium salt. NH4PO3 - H2O = PO2·NH2,
an as yet unknown amide, and PO2·NH2 - H2O gives the nitrile PON.
This relation is confirmed by the fact that PON, moistened with water,
gives metaphosphoric acid when ignited. It is the analogue of nitrous
oxide, NON. It is a very stable compound, more so than the preceding.[29]
[179]
The most important analogue of phosphorus is arsenic, the metallic
aspect of which and the general character of its compounds of the types
AsX3 and AsX5 at once recall the metals. The hydrate of its highest
oxide, arsenic acid (ortho-arsenic acid), H3AsO4, is an oxidising agent,
and gives up a portion of its oxygen to many other substances; but,
nevertheless, it is very like phosphoric acid. Mitscherlich established
the conception of isomorphism by comparing the salts of these acids.[30]
[180]
Arsenic occurs in nature, not only combined with metals, but also,
although rarely, native and also in combination with sulphur in two
minerals—one red, realgar, As2S2, and the other yellow, orpiment,
As2S3 (Chapter XX., Note 2929). Arsenic occurs, but more rarely, in
the form of salts of arsenic acid—for instance, the so-called cobalt and
nickel blooms, two minerals which are found accompanying other cobalt
ores, are the arsenates of these metals. Arsenic is also found in certain
clays (ochres) and has been discovered in small quantities in some
mineral springs, but it is in general of rarer occurrence in nature than
phosphorus. Arsenic is most frequently extracted from arsenical
pyrites, FeSAs, which, when roasted without access of air, evolves the
vapour of arsenic, ferrous sulphide being left behind. It is also obtained
by heating arsenious anhydride with charcoal, in which case carbonic
oxide is evolved. In general, the oxides and other compounds are very
easily reduced. Solid arsenic is a steel-grey brittle metal, having a
bright lustre and scaly structure. Its specific gravity is 5·7. It is
opaque and infusible, but volatilises as a yellow vapour which on cooling
deposits rhombohedral crystals.[30 bis] The vapour density of arsenic is
150 times greater than that of hydrogen—that is, its molecule, like that
of phosphorus, contains 4 atoms, As4. When heated in the air, arsenic
easily oxidises into white arsenious anhydride, As2O3, but even at the
ordinary temperature it loses its lustre (becomes dull), owing to the
formation of a coating of a lower oxide. The latter appears to be as
volatile as arsenious anhydride, and it is probable that it is owing to
the presence of this compound that the vapours of arsenious compounds,
when heated with charcoal (for example, in the reducing flame of a blow-pipe),
have the characteristic smell of garlic, because the vapour of
arsenic itself has not this odour.
Arsenic easily combines with bromine and chlorine;[31] nitric acid[181]
and aqua regia also oxidise it into the higher oxide, or rather its hydrate,
arsenic acid.[32] As far as is known, it does not decompose steam, and[182]
it acts exceedingly slowly on those acids, like hydrochloric, which are
not capable of oxidising.
Arseniuretted hydrogen, arsine, AsH3, resembles phosphuretted
hydrogen in many respects. This colourless gas, which liquefies into a
mobile liquid at -40°, has a disagreeable garlic-like odour, is only
slightly soluble in water, and is exceedingly poisonous. Even in a
small quantity it causes great suffering, and if present to any considerable
amount in air it even causes death. The other compounds of arsenic
are also poisonous, with the exception of the insoluble sulphur compound
and some compounds of arsenic acid. Arseniuretted hydrogen, AsH3,
is obtained by the action of water on the alloy of arsenic and sodium,
sodium hydroxide and arseniuretted hydrogen being formed. It is
also formed by the action of sulphuric acid on the alloy of arsenic and
zinc: Zn3As2 + 3H2SO4 = 2AsH3 + 3ZnSO4.[33] The oxygen compounds
of arsenic are very easily reduced by the action of hydrogen at the
moment of its evolution from acids, and the reduced arsenic then
combines with the hydrogen; hence, if a certain amount of an oxygen
compound of arsenic be put into an apparatus containing zinc and
sulphuric acid (and thus serving for the evolution of hydrogen), the
hydrogen evolved will contain arseniuretted hydrogen. In this case it
is diluted with a considerable amount of hydrogen. But its presence
in the most minute quantities may be easily recognised from the fact
that it is easily decomposed by heat (200° according to Brunn) into
metallic arsenic and hydrogen, and therefore if such impure hydrogen
he passed through a moderately-heated tube metallic arsenic will be
deposited as a bright layer on the part of the tube which was heated
(see Note 30 bis). This reaction is so sensitive that it enables the
most minute traces of arsenic to be discovered; hence it is employed
in medical jurisprudence, as a test in poisoning cases. It is easy to
discover the presence of arsenic in common zinc, copper, sulphuric and
hydrochloric acids, &c. by this method. It is obvious that in testing
for poison by Marsh's apparatus it is necessary to take zinc and sulphuric
acid quite free from arsenic. The arsenic deposited in the tube
may be driven as a volatile metal from one place to another in the
current of hydrogen evolved, owing to its volatility. This forms a
distinction between arseniuretted and antimoniuretted hydrogen, which[183]
is decomposed by heat in just the same way as arseniuretted hydrogen,
but the mirror given by Sb is not so volatile as that formed by As.
Fig. 84.—Formation and decomposition of arseniuretted hydrogen. Hydrogen is evolved in the
Woulfe's bottle, and when the gas comes off, a solution containing arsenic is poured through the
funnel. The presence of AsH3 is recognised from the deposition of a mirror of arsenic when the
gas-conducting tube is heated. If the escaping hydrogen be lighted, and a porcelain dish be held
in the flame, a film of arsenic is deposited on it. The gas is dried by passing through the tube
containing calcium chloride. This apparatus is used for the detection of arsenic by Marsh's test.
If hydrogen contains arseniuretted hydrogen, it also gives metallic
arsenic when it burns, because in the reducing flame of hydrogen the
oxygen attracted combines entirely with the hydrogen and not with
the arsenic, so that if a cold object, such as a piece of china, be held in
the hydrogen flame the arsenic will be deposited upon it as a metallic
spot.[34]
The most common compound of arsenic is the solid and volatile[184]
arsenious anhydride, As2O3, which corresponds with phosphorous and
nitrous anhydrides. This very poisonous, colourless, and sweet-tasting
substance is generally known under the name of arsenic, or white
arsenic. The corresponding hydrate is as yet unknown; its solutions,
when evaporated, yield crystals of arsenious anhydride. It is chiefly
prepared for the dyer, and is also used as a vermin killer, and sometimes
in medicine; it is a product from which all other compounds of arsenic
can be prepared. It is obtained as a by-product in roasting cobalt
and other ores containing arsenic. Arsenical pyrites are sometimes
purposely roasted for the extraction of arsenious anhydride. When
arsenical ores are burnt in the air, the sulphur and arsenic are converted
into the oxides As2O3 and SO2. The former is a solid at the ordinary
temperature, and the latter gaseous, and therefore the arsenious anhydride
is deposited as a sublimate in the cooler portion of the flues
through which the vapours escape from the furnace. It collects in
condensing chambers especially constructed in the flues. The deposit
is collected, and after being distilled gives arsenious anhydride in the
form of a vitreous non-crystalline mass. This is one of the varieties of
arsenious anhydride, which is also known in two crystalline forms.
When sublimed—i.e. when it rapidly passes from the state of vapour
to the solid state—it appears in the regular system in the form of octahedra.[35]
It is obtained in the same form when it is crystallised from
acid solutions. The specific gravity of the crystals is 3·7. The other
crystalline form (in prisms) belongs to the rhombohedral system, and is
also formed by sublimation when the crystals are deposited on a heated
surface, or when it is crystallised from alkaline solutions.[36]
[185]
Solutions of arsenious anhydride have a sweet metallic taste, and
give a feeble acid reaction. Its solubility increases with the admixture
of acids and alkalis. This shows the property of arsenious anhydride
of forming salts with acids and alkalis. And in fact compounds of it
with hydrochloric acid (Note 3131), sulphuric anhydride (see further on),
and with the alkali oxides are known.[37] If silver nitrate be added to
a solution of arsenious anhydride, it does not give any precipitate unless
a certain amount of the arsenious anhydride is saturated with an alkali—for
instance, ammonia. It then gives a precipitate of silver arsenite,
Ag3AsO3. This is yellow, soluble in an excess of ammonia, and anhydrous;
it distinctly shows that arsenious acid is tribasic, and that it
differs in this respect from phosphorous acid, in which only two atoms of
hydrogen can be replaced by metals.[38] The feeble acid character of[186]
arsenious anhydride is confirmed by the formation of saline compounds
with acids. In this respect the most remarkable example is the
anhydrous compound with sulphuric acid, having the composition
As2O3,SO3. It is formed in the roasting of arsenical pyrites in those
spaces where the arsenious anhydride condenses, a portion of the
sulphurous anhydride being converted into sulphuric anhydride, SO3,
at the expense of the oxygen of the air. The compound in question
forms colourless tabular crystals, which are decomposed by water with
formation of sulphuric acid and arsenious anhydride.[39]
Antimony (stibium), Sb = 120, is another analogue of phosphorus.
In its external appearance and the properties of its compounds it resembles
the metals still more closely than arsenic. In fact, antimony
has the appearance, lustre, and many of the characteristic properties of
the metals. Its oxide, Sb2O3, exhibits the earthy appearance of rust
or of lime, and has distinctly basic properties, although it corresponds
with nitrous and phosphorous anhydride, and is able, like them, to give
saline compounds with bases. At the same time antimony presents, in
the majority of its compounds, an entire analogy with phosphorus and
arsenic. Its compounds belong to the type SbX3 and SbX5. It is
found in nature chiefly in the form of sulphide, Sb2S3. This substance
sometimes occurs in large masses in mineral veins and is known in
mineralogy under the name of antimony glance or stibnite, and commercially
as antimony (Chapter XX., Note 29). The most abundant
deposits of antimony ore occur in Portugal (near Oporto on the Douro).
Besides which antimony partially or totally replaces arsenic in some
minerals; thus, for example, a compound of antimony sulphide and
arsenic sulphide with silver sulphide is found in red silver ore. But in
every case antimony is a rather rare metal found in few localities. In
Russia it is known to occur in Daghestan in the Caucasus. It is
extracted chiefly for the preparation of alloys with lead and tin,
which are used for casting printing type.[40] Some of its compounds are[187]
also used in medicine, the most important in this respect being antimony
pentasulphide, Sb2S5 (sulfur auratum antimonii), and tartar emetic,
which is a double salt derived from tartaric acid and has the composition
C4H4K(SbO)O6. Even the native antimony sulphide is used in
large quantities as a purgative for horses and dogs. Metallic antimony
is extracted from the glance, Sb2S2, by roasting, when the sulphur
burns away and the antimony oxidises, forming the oxide Sb2O3, which
is then heated with charcoal, and thus reduced to a metallic state. The
reduction may be carried on in the laboratory on a small scale by
fusing the sulphide with iron which takes up the sulphur.[40 bis]
Metallic antimony has a white colour and a brilliant lustre; it
remains untarnished in the air, for the metal does not oxidise at the
ordinary temperature. It crystallises in rhombohedra, and always
shows a distinctly crystalline structure which gives it quite a different
aspect from the majority of the metals yet known. It is most like
tellurium in this respect. Antimony is brittle, so that it is very easily
powdered; its specific gravity is 6·7, it melts at about 432°, but only
volatilises at a bright red heat. When heated in the air—for instance,
before the blow-pipe—it burns and gives white odourless fumes, consisting
of the oxide. This oxide is termed antimonious oxide, although
it might as well be termed antimonious anhydride. It is given the
first name because in the majority of cases its compounds with acids
are used, but it forms compounds with the alkalis just as easily.
Antimonious oxide, like arsenious anhydride, crystallises either in
regular octahedra or in rhombic prisms; its specific gravity is 5·56; when
heated it becomes yellow and then fuses, and when further heated in air
it oxidises, forming an oxide of the composition Sb2O4. Antimonious
oxide is insoluble in water and in nitric acid, but it easily dissolves in
strong hydrochloric acid and in alkalis, as well as in tartaric acid or
solutions of its acid salts. When dissolved in the latter it forms tartar
emetic. It is precipitated from its solutions in alkalis and acids (by[188]
the action of acids on the former and alkalis on the latter). It occurs
native but rarely. As a base it gives salts of the type SbOX (as if the
basic salts = SbX3, Sb2O3) and hardly ever forms salts, SbX3. In the
antimonyl salts, SbOX, the group SbO is univalent, like potassium or
silver. The oxide itself is (SbO)2O, the hydroxide, SbO(OH), &c.;
tartar emetic is a salt in which one hydrogen of tartaric acid is replaced
by potassium and the other by antimonyl, SbO. Antimonious oxide is
very easily separated from its salts by any base, but it must be observed
that this separation does not take place in the presence of tartaric acid,
owing to the property of tartaric acid of forming a soluble double salt—i.e.
tartar emetic.[41]
If metallic antimony, or antimonious oxide, be oxidised by an excess
of nitric acid and the resultant mass be carefully evaporated to dryness,
metantimonic acid, SbHO3, is formed. Its corresponding potassium
salt, 2SbKO3,5H2O, is prepared by fusing metallic antimony with
one-fourth its weight of nitre and washing the resultant mass with
cold water. This potassium salt is only slightly soluble in water (in
50 parts) and the sodium salt is still less so. An ortho-acid, SbH3O4,
also appears to exist;[41 bis] it is obtained by the action of water on
antimony pentachloride, but it is very unstable, like the pentachloride,
SbCl5, itself, which easily gives up Cl2, leaving antimony trichloride,
SbCl3, and this is decomposed by water, forming an oxychloride—SbOCl,
only slightly soluble in water. When antimonic acid is heated[189]
to an incipient red heat, it parts with water and forms the anhydride,
Sb2O5, of a yellow colour and specific gravity 6·5.[42]
The heaviest analogue of nitrogen and phosphorus is bismuth,[190]
Bi = 208. Here, as in the other groups, the basic, metallic, properties
increase with the atomic weight. Bismuth does not give any
hydrogen compound and the highest oxide, Bi2O5, is a very feeble
acid oxide. Bismuthous oxide, Bi2O3, is a base, and bismuth itself
a perfect metal. To explain the other properties of bismuth it must
further be remarked that in the eleventh series it follows mercury,
thallium and lead, whose atomic weights are near to that of bismuth,
and that therefore it resembles them and more especially its nearest
neighbour, lead. Although PbO and PbO2, represent types different
from Bi2O3 and Bi2O5, they resemble them in many respects, even in
their external appearance, moreover the lower oxides both of Pb and Bi
are basic and the higher acid, which easily evolve oxygen. But judging
by the formula, Bi2O3 is a more feeble base than PbO. They both easily
give basic salts.
Bismuth forms compounds of two types, BiX3 and BiX5,[43] which
entirely recall the two types we have already established for the
compounds of lead. Just as in the case of lead, the type PbX2, is
basic, stable, easily formed, and passes with difficulty into the higher
and lower types, which are unstable, so also in the case of bismuth the
type of combination BiX3 is the usual basic form. The higher type of
combination, BiX5,[44] in fact behaves toward this stable type, BiX3, in
exactly the same manner as lead dioxide does to the monoxide; and
bismuthic acid is obtained by the action of chlorine on bismuth oxide
suspended in water, in exactly the same way as lead dioxide is obtained[191]
from lead oxide. It is an oxidising agent like lead dioxide, and even
the acid character in bismuthic acid is only slightly more developed
than in lead dioxide. Here, as in the case of lead (minium), intermediate
compounds are easily formed in which the bismuth of the
lower oxide plays the part of a base combined with the acid which is
formed by the higher form of the oxidation of bismuth.
Fig. 85.—Furnace used for the extraction of bismuth
from its ores.
In nature, bismuth occurs in only a few localities and in small
quantities, most frequently in a native state, and more rarely as oxide
and as a compound of bismuth sulphide with the sulphides of other
metals, and sometimes in gold ores. It is extracted from its native ores
by simple fusion in the furnace shown in fig. 85. This furnace contains
an inclined iron retort, into
the upper extremity of
which the ore is charged,
and the molten metal flows
from the lower extremity.
It is refined by re-melting,
and the pure metal may be
obtained by dissolving in
nitric acid, decomposing the
resultant salt with water,
and reducing the precipitate
by heating it with charcoal.
Bismuth is a metal which
crystallises very well from a molten state. Its specific gravity is 9·8;
it melts at 269°, and if it be melted in a crucible, allowed to cool slowly,
and the crust broken and the remaining molten liquid poured out, perfect
rhombohedral crystals of bismuth are obtained on the sides of the
crucible.[44 bis] It is brittle, has a grey-coloured fracture with a reddish
lustre, is not hard, and is but very slightly ductile and malleable; it
volatilises at a white heat and easily oxidises. It recalls antimony and
lead in many of its properties. When oxidised in air, or when the
nitrate is ignited, bismuth forms the oxide, Bi2O3, as a white powder
which fuses when heated and resembles massicot. The addition of an
excess of caustic potash to a solution of a bismuthous salt gives a white
precipitate of the hydroxide, BiO(OH), which loses its water and gives[192]
the anhydrous oxide when boiled with a solution of caustic potash.
Both the hydroxide and oxide easily dissolve in acids and form bismuthous
salts.
Bismuthous oxide, Bi2O3, is a feeble and unenergetic base. The
normal hydroxide of the oxide Bi2O3 is Bi(OH)3; it parts with water
and forms a metahydroxide (bismuthyl hydroxide), BiO(OH). Both of
these hydroxides have their corresponding saline compounds of the
composition BiX3 and BiOX. And the form BiOX is nothing else but
the type of the basic salt, because 3ROX = RX + R2O3. It is evident
that in the type BiX3 the bismuth replaces three atoms of hydrogen.
And indeed with phosphoric acid solutions of the bismuthous salts
give a precipitate of the composition BiPO4. On the other hand, in
the form of compounds BiOX or Bi(OH)2X, the univalent group (BiO)
or (BiH2O2) is combined with X. Many bismuth salts are formed
according to the type BiOX. For instance the carbonate, (BiO)2CO3,
which corresponds with the other carbonates M2CO3. It is obtained
as a white precipitate when a solution of sodium carbonate is added to
a solution of a bismuth salt.[45] The compound radicle BiO is not a
special natural grouping, as it was formerly represented to be; it is
simply a mode of expression for showing the relation between the compound
in question and the compounds of other oxides.
Three salts of nitric acid are known containing bismuthous
oxide. If metallic bismuth or its oxide be dissolved in nitric acid, it
forms a colourless transparent solution containing a salt which separates
in large transparent crystals containing Bi(NO3)3,5H2O. When
heated at 80° these crystals melt in their water of crystallisation, and
in so doing lose a portion of their nitric acid together with water,
forming a salt whose empirical formula is Bi2N2H2O9. If the preceding
salt belongs to the type BiX3, this one should belong to the form
BiOX, because it = BiO(NO3) + Bi(H2O2)(NO3). This salt may be
heated to 150° without change. When the first colourless crystalline
salt dissolves in water it is decomposed. There is no decomposition if
an excess of acid be added to the water—that is to say, the salt is able
to exist in an acid solution without decomposing, without separation of
the so-called basic salt—but by itself it cannot be kept in solution; water
decomposes this salt, acting on it like an alkali. In other words the
basic properties of bismuthic oxide are so feeble that even water acts by
taking up a portion of the acid from it. Here we see one of the most
striking facts, long since observed, confirming that action of water on
salts about which we have spoken in Chapter X. and elsewhere. This[193]
action on water may be expressed thus:—BiX3 + 2H2O = Bi(OH)2X
+ 2XH. A salt of the type Bi(OH)2X is obtained in the precipitate.
But if the quantity of acid, HX, be increased, the salt BiX3 is
again formed and passes into solution. The quantity of the salt BiOX
which passes into solution on the addition of a given quantity of acid
depends indisputably on the amount (mass) of water (Muir). The
solution, which is perfectly transparent with a small amount of water,
becomes cloudy and deposits the salt of the type BiOX, when
diluted. The white flaky precipitate of Bi(OH)2NO3 formed from the
normal salt Bi(NO3)3 by mixing it with five parts of water, and in
general with a small amount of water, is used in medicine under the
name of magistery of bismuth.[46]
Metallic bismuth is used in the preparation of fusible alloys. The
addition of bismuth to many metals renders them very hard, and at the
same time generally lowers their melting point to a considerable extent.
Thus Wood's metal, which contains one part of cadmium, one part of
tin, two parts of lead, and four parts of bismuth, fuses at about 60°, and
in general many alloys composed of bismuth, tin, lead, and antimony
melt below or about the boiling point of water.[47]
[194]
Just as in group II., side by side with the elements zinc, cadmium,
and mercury in the uneven series, we found calcium, strontium, and
barium in the even series; and as in group IV., parallel to silicon, germanium,
tin, and lead, we noticed thallium, zirconium, cerium, and
thorium; so also in group V. we find, beside those elements of the uneven
series just considered by us, a series of analogues in the even series,
which, with a certain degree of similarity (mainly quantitative, or relative
to the atomic weights), also present a series of particular (qualitative)
independent points of distinction. In the even series are known vanadium,
which stands between titanium and chromium, niobium, between
zirconium and molybdenum, and tantalum, situated near tungsten (an
element of group VI. like chromium and molybdenum). Just as bismuth
is similar in many respects to its neighbour lead, so also do these neighbouring
elements resemble each other, even in their external appearance,
not to mention the quality of their compounds, naturally taking into
account the differences of type corresponding with the different groups.
The occurrence in group V. determines the type of the oxides, R2O3
and R2O5, and the development of an acid character in the higher
oxides. The occurrence in the even series determines the absence of
volatile compounds, RH3, for these metals, and a more basic character
of the oxides of a given composition than in the uneven series, &c.[48]
Vanadium, niobium, and tantalum belong to the category of rare metals,
and are exceedingly difficult to obtain pure, more especially owing to
their similarity to, and occurrence with, chromium, tungsten and other
metals, and also in combination among themselves; therefore it is
natural that they have been far from completely studied, although since
1860 chemists have devoted not a little time to their investigation.
The researches carried out by Marignac, at Geneva, on niobium,
and by Sir Henry Roscoe, at Manchester, on vanadium deserve
special attention. The undoubted external resemblance of the compounds
of chromium and vanadium, as well as the want of completeness
in the knowledge of the compounds of vanadium, long caused
its oxides to be considered analogous in atomic composition to those
formed by chromium. The higher oxide of vanadium was therefore
supposed to have the formula VO3. But the fact of the matter is,
that the chemical analogy of the elements does not hold in one
direction only; vanadium is at one and the same time the analogue of[195]
chromium, and consequently of the elements like sulphur of group VI,
and also the analogue of phosphorus, arsenic, and antimony; just as
bismuth stands in respect to lead and antimony. Investigation has
shown that the compounds of vanadium are always accompanied by those
of phosphorus as well as of iron, and that it is even more difficult to
separate it from the compounds of phosphorus than from those of iron
and tungsten. We should have to extend our description considerably
if we wished to give the complete history, even of vanadium alone, not
to mention niobium and tantalum, all the more as questions would not
unfrequently arise concerning the compounds of these elements which
have not yet been fully elucidated. We shall therefore limit ourselves
to pointing out the most important features in the history of these
elements, the more so since the minerals themselves in which they occur
are exceedingly rare and only accessible to a few investigators.
An important point in the history of the members of this group
is the circumstance that they form volatile compounds with chlorine,
similar to the compounds of the elements of the phosphorus group,
namely, of the type RX5. The vapour densities of the compounds
of this kind were determined, and served as the most important
basis for the explanation of the atomic composition of these molecules.
In this we see the power of general and fundamental laws, like the
law of Avogadro-Gerhardt. An oxychloride, VOCl3, is known for
vanadium, which is the perfect analogue of phosphorus oxychloride.
It was formerly considered to be vanadium chloride, for just as in
the case of uranium (Chapter XXI.), its lower oxide, VO, was considered
to be the metal, because it is exceedingly difficultly reduced—even
potassium does not remove all the oxygen, besides which it has
a metallic appearance, and decomposes acids like a metal; in a word, it
simulates a metal in every respect. Vanadium oxychloride is obtained
by heating the trioxide, V2O3, mixed with charcoal, in a current of hydrogen;
the lower oxide of vanadium is then formed, and this, when
heated in a current of dry chlorine, gives the oxychloride VOCl3 as a
reddish liquid which does not act on sodium and may be purified by
distillation over this metal. It fumes in the air, giving reddish vapours;
it reacts on water, forming hydrochloric and vanadic acids; hence,
on the one hand it is very similar to phosphorus oxychloride, and on
the other hand to chromium oxychloride, CrO2Cl2 (Chapter XXI.).
It is of a yellow colour, its specific gravity is 1·83, it boils at 120°, and
its vapour density is 86 with respect to hydrogen; therefore the above
formula expresses its molecular weight.[49]
[196]
Vanadic anhydride, V2O5, is obtained either in small quantities
from certain clays where it accompanies the oxides of iron (hence some
sorts of iron contain vanadium) and phosphoric acid, or from the rare
minerals: volborthite, CuHVO4, or basic vanadate of copper; vanadinite,
PbCl23Pb3(VO4)2; lead vanadate, Pb3(VO4)2, &c. The latter
salts are carefully ignited for some time with one-third of their weight of
nitre; the fused mass thus formed is powdered and boiled in water: the
yellow solution obtained contains potassium vanadate. The solution
is neutralised with acid, and barium chloride added; a meta-salt,
Ba(VO3)2, is then precipitated as an almost insoluble white powder,
which gives a solution of vanadic acid when boiled with sulphuric
acid. (The precipitate is at first yellow, as long as it remains amorphous,
but it afterwards becomes crystalline and white.) The solution
thus obtained is neutralised with ammonia, which thus forms ammonium
(meta) vanadate, NH4VO3, which, when evaporated, gives colourless
crystals, insoluble in water containing sal-ammoniac; hence this
salt is precipitated by adding solid sal-ammoniac to the solution.
Ammonium vanadate, when ignited, leaves vanadic acid behind. In
this it differs from the corresponding chromium salt, which is deoxidised
into chromium oxide when ignited. In general, vanadic acid
has but a small oxidising action. It is reduced with difficulty, like
phosphoric or sulphuric acid, and in this differs from arsenic and
chromic acids. Vanadic acid, like chromic acid, separates from its solution
as the anhydride V2O5, and not in a hydrous state. Vanadic
anhydride, V2O5, forms a reddish-brown mass, which easily fuses and re-solidifies
into transparent crystals having a violet lustre (another point
of resemblance to chromic acid); it dissolves in water, forming a yellow
solution with a slightly acid reaction.[50]
[197]
Niobium and tantalum[51] occur as acids in rare minerals, and are
mainly extracted from tantalite and columbite, which are found in
Bavaria, Finland, North America, and in the Urals. These minerals
are composed of the ferrous salts of niobic and tantalic acids; they[198]
contain about 15 per cent. of ferrous oxide in isomorphous mixture
with manganous oxide, in combination with various proportions of
tantalic and niobic anhydrides. These minerals are first fused with
a considerable amount of potassium bisulphate, and the fused mass
is boiled in water, which dissolves the ferrous and potassium salts and
leaves an insoluble residue of impure niobic and tantalic acids. This
raw product is then treated with ammonium sulphide, in order to
extract the tin and tungsten, which pass into solution. The residue
containing the acids (according to Marignac) is then treated with hydrofluoric
acid, in which it entirely dissolves, and potassium fluoride is
added to the resultant hot solution; on cooling, a sparingly soluble
double fluoride of potassium and tantalum separates out in fine crystals,
while the much more soluble niobium salt remains in solution. The
difference in the solubility of these double salts in water acidified with
hydrofluoric acid (in pure water the solution becomes cloudy after a
certain time) is so great that the tantalum compound requires 150 parts
of water for its solution, and the niobium compound only 13 parts.
The Greenland columbite (specific gravity 5·36) only contains niobic
acid, and that from Bodenmais, Bavaria (specific gravity 6·06) almost
equal quantities of tantalic and niobic acids. Having isolated tantalic
and niobic salts, Marignac found that the relation between the potassium
and fluorine in them is very variable—that is, that there exist
various double salts of fluoride of potassium, and of the fluorides of
the metals of this group, but that with an excess of hydrofluoric acid
both the tantalum and niobium compounds contain seven atoms of
fluorine to two of potassium, whence it must be concluded that the
simplest formula for these double salts will be K2RF7 = RF5,2KF;
that is, that the type of the higher compounds of niobium and tantalum
is RX5, and hence is similar to phosphoric acid. A chloride, TaCl5,
may be obtained from pure tantalic acid by heating it with charcoal
in a current of chlorine. This is a yellow crystalline substance, which
melts at 211°, and boils at 241°; its vapour density with respect to
hydrogen is 180, as would follow from the formula TaCl5. It is completely
decomposed by water into tantalic and hydrochloric acids.
Niobium pentachloride may be prepared in the same manner; it fuses
at 194°, and boils at 240°. When treated with water this substance
gives a solution containing niobic acid, which only separates out on
boiling the solution. Delafontaine and Deville found its vapour
density to be 9·3 (air = 1), as is shown by its formula NbCl5.[52]
[200]
CHAPTER XX
SULPHUR, SELENIUM, AND TELLURIUM
The acid character of the higher oxides RO3 of the elements of
group VI. is still more clearly defined than that of the higher oxides of
the preceding groups, whilst feeble basic properties only appear in
the oxides RO3 of the elements of the even series, and then only for
those elements having a high atomic weight—that is, under those two
conditions in which, as a rule, the basic characters increase. Even the
lower types RO2 and R2O3, &c., formed by the elements of group VI.,
are acid anhydrides in the uneven series, and only those of the elements
of the even series have the properties of peroxides or even of bases.
Sulphur is the typical representative of group VI., both on account of
the fact that the acid properties of the group are clearly defined in it, and
also because it is more widely distributed in nature than any of the other
elements belonging to this group. As an element of the uneven series
of group VI., sulphur gives H2S, sulphuretted hydrogen, SO3, sulphuric
anhydride, and SO2, sulphurous anhydride. And in all of them we find
acid properties—SO3 and SO2 are anhydrides of acids, and H2S is an
acid, although a feeble one. As an element sulphur has all the properties
of a true non-metal; it has not a metallic lustre, does not
conduct electricity, is a bad conductor of heat, is transparent, and
combines directly with metals—in short it has all the properties of the
non-metals, like oxygen and chlorine. Furthermore, sulphur exhibits
a great qualitative and quantitative resemblance to oxygen, especially
in the fact that, like oxygen, it combines with two atoms of hydrogen,
and forms compounds resembling oxides with metals and non-metals.
From this point of view sulphur is bivalent, if the halogens are univalent.[1]
The chemical character of sulphur is expressed by the fact[201]
that it forms a very slightly stable and feebly energetic acid with
hydrogen. The salts corresponding with this acid are the sulphides,
just as the oxides correspond to water and the chlorides to hydrochloric
acid. However, as we shall afterwards see more fully, the sulphides
are more analogous to the former than to the latter. But although
combining with metals, like oxygen, sulphur also forms chemically
stable compounds with oxygen, and this fact impresses a peculiar
character on all the relations of this element.[2]
Sulphur belongs to the number of those elements which are very
widely distributed in nature, and occurs both free and combined in
various forms. The atmosphere, however, is almost entirely free from
compounds of sulphur, although a certain amount of them should be
present, if only from the fact that sulphurous anhydride is emitted
from the earth in volcanic eruptions, and in the air of cities, where
much coal is burnt, since this always contains FeS2. Sea and river
water generally contain more or less sulphur in the form of sulphates.
The beds of gypsum, sodium sulphate, magnesium sulphate,
and the like are formations of undoubtedly aqueous origin. The sulphates
contained in the soil are the source of the sulphur found in
plants, and are indispensable to their growth. Among vegetable
substances, the proteïds always contain from one to two per cent. of
sulphur. From plants the albuminous substances, together with their
sulphur, pass into the animal organism, and therefore the decomposition
of animal matter is accompanied by the odour of sulphuretted hydrogen,
as the product into which the sulphur passes in the decomposition of
the albuminous substances. Thus a rotten egg emits sulphuretted hydrogen.
Sulphur occurs largely in nature, as the various insoluble sulphides
of the metals. Iron, copper, zinc, lead, antimony, arsenic, &c.,
occur in nature combined with sulphur. These sulphides frequently
have a metallic lustre, and in the majority of cases occur crystallised,[202]
and also very often several sulphides occur combined or mixed
together in these crystalline compounds. If they are yellow and have
a metallic lustre they are called pyrites. Such are, for example,
copper pyrites, CuFeS2, and iron pyrites, FeS2, which is the commonest
of all. They are all also known as glances or blendes if they are
greyish and have a metallic lustre—for example, zinc blende, lead
glance, PbS, antimony glance, Sb2S3, &c. And, lastly, sulphur occurs
native. It occurs in this form in the most recent geological formations
in admixture with limestone and gypsum, and most frequently in the
vicinity of active or extinct volcanoes. As the gases of volcanoes contain
sulphur compounds—namely, sulphuretted hydrogen and sulphurous
anhydride, which by reacting on one another may produce sulphur, which
also frequently appears in the craters of volcanoes as a sublimate—it
might be imagined that the sulphur was of volcanic origin. But on a
nearer acquaintance with its mode of occurrence, and more especially
considering its relation to gypsum, CaSO4, and limestone, the present
general opinion leads to the conclusion that the ‘native’ sulphur has
been formed by the reduction of the gypsum by organic matter and
that its occurrence is only indirectly connected with volcanic agencies.
Near Tetush, on the Volga, there are beds containing gypsum, sulphur,
and asphalt (mineral tar). In Europe the most important deposits of
sulphur are in the south of Sicily from Catania to Girgenti.[3] There
are very rich deposits of sulphur in Daghestan near Cherkai and
Cherkat in Khyut, near Mount Kanabour-bam, near Petrovsk, and
in the Kira Koumski steppes in the Trans-Caspian provinces, which
are able to supply the whole of Russia with this mineral. Abundant
deposits of sulphur have also been found in Kamtchatka in the neighbourhood
of the volcanoes. The method of separation of the sulphur
from its earthy impurities is based on the fact that sulphur melts when
it is heated. The fusion is carried on at the expense of a portion of
the sulphur, which is burnt, so that the remainder may melt and run
from the mass of the earth. This is carried on in special furnaces
called calcaroni, built up of unhewn stone in the neighbourhood of the
mines.[4]
[203]
Fig. 86.—Refining sulphur by sublimation.
Sulphur is purified by distillation in special retorts (see fig. 86) by
passing the vapour into a chamber G built of stone. The first portions
of the vapour entering into the condensing chamber are condensed
straightway from the vapour into a solid state, and form a fine powder
known as flowers of sulphur.[5] But when the temperature of the
receiver attains the melting point of sulphur, it passes into a liquid[204]
state and is cast into moulds (like sealing wax), and is then known
under the name of roll sulphur.[6]
In an uncombined state sulphur exists in several modifications, and
forms a good example of the facility with which an alteration of properties
can take place without a change of composition—that is, as regards
the material of a substance. Common sulphur has the well-known
yellow colour. This colour fades as the temperature falls, and at -50°
sulphur is almost colourless. It is very brittle, so that it may be easily
converted into a powder, and it presents a crystalline structure, which,
by the way, shows itself in the unequal expansion of lumps of sulphur
by heat. Hence when a piece of sulphur is heated by the warmth of
the hand, it emits sounds and sometimes cracks, which probably also
depends on the bad heat-conducting power of this substance. It is easily
obtained in a crystalline form by artificial means, because although
insoluble in water it dissolves in carbon bisulphide, and in certain oils.[7]
Solutions of sulphur in carbon bisulphide when evaporated at the
ordinary temperature yield well-formed transparent crystals of sulphur
in the form of rhombic octahedra, in which form it occurs native. The
specific gravity of these crystals is 2·045. Fused sulphur, cast into
moulds and cooled, has, after being kept a long time, a specific gravity
2·066; almost the same as that of the crystalline sulphur of the above
form, which shows that common sulphur is the same as that which[205]
crystallises in octahedra. The specific heat of octahedral sulphur is
0·17; it melts at 114°, and forms a bright yellow mobile liquid. On
further heating, the fused sulphur undergoes an alteration, which we
shall presently describe, first observing that the above octahedral state
of sulphur is its most stable form. Sulphur may be kept at the ordinary
temperature in this form for an indefinite length of time, and
many other modifications of sulphur pass into this form after being left
for a certain time at ordinary temperature.
If sulphur be melted and then slightly cooled, so that it forms a
crust on the surface and over the sides of the crucible, while the
internal mass remains liquid, then the sulphur takes another crystalline
form as it solidifies. This may be seen by breaking the crust, and
pouring out the remaining molten sulphur.[8] It is then found that the
sides of the crucible are covered with prismatic crystals of the monoclinic
system; they have a totally different appearance from the above-described
crystals of rhombic sulphur. The prismatic crystals are
brown, transparent, and less dense than the crystals of rhombic
sulphur, their specific gravity being only 1·93, and their melting point
higher—about 120°. These crystals of sulphur cannot be kept at
the ordinary temperature, which is indeed evident from the fact that
in time they turn yellow; the specific gravity also changes, and they
pass completely into the ordinary modification. This is accompanied
by a considerable development of heat, so that the temperature of the
mass may rise 12°. Thus sulphur is dimorphous—that is, it exists in
two crystalline forms, and in both forms it has independent physical
properties. However, no chemical reactions are known which distinguish
the two modifications of sulphur, just as there are none distinguishing
aragonite from calcspar.[9]
If molten sulphur be heated to 158° it loses its mobility and
becomes thick and very dark-coloured, so that the crucible in which it[206]
is heated may be inverted without the sulphur running out. When
heated above this temperature the sulphur again becomes liquid, and
at 250° it is very mobile, although it does not acquire its original
colour, and at 440° it boils. These modifications in the properties of
sulphur depend not only on the variations of temperature, but also on
a change of structure. If sulphur, heated to about 350°, be poured in
a thin stream into cold water, it does not solidify into a solid mass, but
retains its brown colour and remains soft, may be stretched out into
threads, and is elastic, like guttapercha. But in this soft and ductile
state, also, it does not remain for a long time. After the lapse of a
certain period this soft transparent sulphur hardens, becomes opaque,
passes into the ordinary yellow modification of sulphur, and in so doing
develops heat, just as in the conversion of the prismatic into the octahedral
variety. The soft sulphur is characterised by the fact that a
certain portion of it is insoluble in carbon bisulphide. When soft sulphur
is immersed in this liquid, only a portion of common sulphur
passes into solution, whilst a certain portion is quite insoluble and
remains so for a long time. The maximum proportion of insoluble
sulphur is obtained by heating slightly above 170°. It melts at 114°.
An exactly similar insoluble amorphous sulphur is obtained in certain
reactions in the wet way, when sulphur separates out from solutions.
Thus sodium thiosulphate, Na2S2O3, when treated with acids, gives a
precipitate of sulphur, which is insoluble in carbon bisulphide. The
action of water on sulphur chloride also gives a similar modification of
sulphur. Certain sulphides, when treated with nitric acid, also yield
sulphur in this form.[10]
[207]
At temperatures of 440° to 700° the vapour density of sulphur is
6·6 referred to air—i.e. about 96 referred to hydrogen. Hence, at
these temperatures the molecule of sulphur contains six atoms, it has
the composition S6. The agreement between the observations of Dumas,
Mitscherlich, Bineau, and Deville confirms the accuracy of this result.
But in this respect the properties of sulphur were found to be variable.
When heated to higher temperatures, that is to say, above 800°, the
vapour density of sulphur is found to be one-third of this quantity, i.e.
about 32 referred to hydrogen. At this temperature the molecule of
sulphur, like that of hydrogen, oxygen, nitrogen, and chlorine, contains
two atoms; hence the molecular formula is then S2. This variation in
the vapour density of sulphur evidently corresponds with a polymeric
modification, and may be likened to the transformation of ozone, O3,
into oxygen, O2, or better still, of benzene, C6H6, into acetylene,
C2H2.[11]
[208]
In its faculty for combination, sulphur most closely resembles oxygen
and chlorine; like them, it combines with nearly all elements, with
the development of heat and light, forming sulphur compounds, but
as a rule this only takes place at a high temperature. At the ordinary
temperature it does not enter into reactions, owing, amongst other
things, to the fact that it is a solid. In a molten state it acts on most
metals and on the halogens. It burns in air at about 300°, and with
carbon at a red heat, but it does not combine with nitrogen.
Fine wires, or the powders of the greater number of metals, burn in
the vapour of sulphur. The direct combination of hydrogen with sulphur
is restricted by a limit—that is, at a given temperature and under
other given conditions it does not proceed unrestrictedly; there is no
explosion or recalescence. Sulphuretted hydrogen, H2S, decomposes at
its temperature of combination—that is, it is easily dissociated.[12] The
same phenomenon is repeated here as with water, except that the temperatures
at which the attraction of hydrogen for sulphur begins and
ceases are much lower than in the case of oxygen and hydrogen. The
temperature at which combination takes place is here, as in many other
instances, nearly the same as that at which dissociation begins. Hence
sulphuretted hydrogen is formed in a small quantity by the direct
ignition of a mixture of the vapour of sulphur and hydrogen. However,
the temperature must not be high, because otherwise the whole
of the sulphuretted hydrogen is decomposed; but at lower temperatures
a small amount of sulphuretted hydrogen is formed by direct combination.[13]
Sulphuretted hydrogen however, like all other hydrogen compounds,[209]
may be easily obtained by the double decomposition of its corresponding
metallic compounds, the replacement of the metal by hydrogen
being effected by the action of acids on the sulphides. The metallic sulphides
are, as a rule, easily formed. A sulphide, when mixed with a non-volatile
acid, may give, by double decomposition, a salt of the acid taken
and sulphuretted hydrogen, M2S + H2SO4 = H2S + M2SO4. However, it
is not all sulphides nor solutions of all acids that will evolve sulphuretted
hydrogen, which fact is exceedingly characteristic, because, for example,
all carbonates evolve carbonic anhydride when treated with any acid.
Sulphuric acid will only evolve sulphuretted hydrogen from those sulphides
which contain a metal capable of decomposing the acid with the
evolution of hydrogen. Thus zinc, iron, calcium, magnesium, manganese,
potassium, sodium, &c., form sulphides which evolve sulphuretted
hydrogen when treated with sulphuric acid, and the metals themselves
evolve hydrogen with acids.[14] The sulphides of those metals which do
not liberate hydrogen from acids do not generally act on acids—that is,[210]
do not form sulphuretted hydrogen with them; such are, for example,
the sulphides of lead, silver, copper, mercury, tin, &c. Therefore, the
modus operandi of the formation of sulphuretted hydrogen by the action
of acids on metallic sulphides may be looked on as a phenomenon of the
combination of hydrogen, at the moment of its evolution, with the sulphur,
which is combined with the metal. Such a representation is all
the more simple as all the circumstances under which sulphuretted
hydrogen is formed are exactly similar to the conditions of the formation
of hydrogen itself. Thus the usual mode of preparing sulphuretted
hydrogen is by the action of sulphuric acid on ferrous sulphide, in
which the same apparatus and method are employed as in the preparation
of hydrogen, only replacing the metallic iron or zinc by ferrous
sulphide or zinc sulphide. The reaction between sulphide of iron and
sulphuric acid takes place at the ordinary temperature, and is accompanied
by just as small a development of heat as in the liberation of
hydrogen itself, FeS + H2SO4 = FeSO4 + H2S.[15]
In nature sulphuretted hydrogen is formed in many ways. The
most usual mode of its formation is by the decomposition of albuminous
substances containing sulphur, as mentioned above. Another
method is by the reducing action of organic matter on sulphates,
and by the action of water and carbonic acid on the sulphides formed
by this reduction. Volcanic eruptions are a third source of sulphuretted
hydrogen in nature. Although sulphuretted hydrogen is formed in
small quantities everywhere, it nevertheless soon disappears from the
atmosphere, owing to its being easily decomposed by oxidising agencies.
Many mineral waters contain sulphuretted hydrogen, and smell of it;
they are called ‘sulphur waters.’
Sulphuretted hydrogen, at the ordinary temperature, is a colourless
gas, having a very unpleasant odour. It has, as its composition H2S
shows, a specific gravity seventeen times greater than hydrogen, and[211]
therefore it is somewhat heavier than air. Sulphuretted hydrogen liquefies
at about -74°, and at the ordinary temperature when subjected to a
pressure of 10 to 15 atmospheres; at -85° it is converted into a solid
crystalline mass.[15 bis] The easy liquefaction of sulphuretted hydrogen
is evidently allied to its solubility. One volume of water at 0° dissolves
4·37 volumes of sulphuretted hydrogen, at 10° 3·58 volumes,
and at 20° 2·9 volumes.[16] The solutions impart a very feeble red
coloration to litmus paper. This gas is poisonous. One part in fifteen
hundred parts of air will kill birds. Mammalia die in an atmosphere
containing 1⁄200 of this gas.
Sulphuretted hydrogen is very easily decomposed into its component
parts by the action of heat or a series of electric sparks. Hence it is
not surprising that sulphuretted hydrogen undergoes change under the
action of many substances having a considerable affinity for hydrogen
and oxygen. Very many metals[17] evolve hydrogen with sulphuretted
hydrogen, so that in this respect it presents the property of an acid; for
instance, 2H2S + Sn = 2H2 + SnS2. This may be taken advantage of for
determining the composition of sulphuretted hydrogen, because a given
volume then leaves the same volume of hydrogen. On the other hand,
oxygen,[18] chlorine,[19] and even iodine decompose sulphuretted hydrogen,[212]
removing the hydrogen from it and leaving free sulphur, so that in
this reaction the sulphur is replaced by the above-named elements; for
example, H2S + Br2 = 2HBr + S. In no other hydrogen compound is
it so easy to show the substitution, both of hydrogen and of the element
combined with it, as in hydrogen sulphide. This clearly proves
the feeble union between the elements forming this gas. Compounds
containing a considerable amount of oxygen, with which they easily
part, can accomplish the separation of the sulphur very easily. Such
are, for instance, nitrous acid, chromic acid, and even ferric oxide and
the higher oxides like it. Thus, if sulphuretted hydrogen be passed
into a solution of chromic acid or an acid solution of ferric oxide,
water is formed, and the sulphur is separated in a free state. Thus,
sulphuretted hydrogen acts as a reducing agent, in virtue of the hydrogen
it contains. Salts of iodic, chlorous, chloric, and other acids are
reduced by sulphuretted hydrogen, their oxygen acting mainly on its
hydrogen; but in the presence of an excess of a powerful oxidising
agent a portion of the sulphur may also be oxidised to sulphurous
anhydride. The reducing action of sulphuretted hydrogen is frequently
applied in chemical manipulations for the preparation of lower oxides,
and for the conversion of certain oxygen compounds into hydrogen
compounds: thus, the higher oxides of nitrogen are converted into
ammonia by it, and in the presence of alkalis the nitro-compounds are
converted into ammonia derivatives. The reaction of sulphuretted
hydrogen on sulphurous anhydride belongs to this class of phenomena,
the chief products of which are sulphur and water, 2H2S + SO2
= 2H2O + S3.
The acid character of sulphuretted hydrogen is clearly seen in its
action on alkalis and salts.[19 bis] Thus lead oxide and its salts in
the presence of sulphuretted hydrogen form water or an acid, and sulphide
of lead: PbX2 + H2S = PbS + 2HX. This reaction takes place
even in the presence of powerful acids, because lead sulphide is one of
those sulphides which are unacted on by acids, and in solutions the
reaction is a complete one. This reaction is taken advantage of for
the preparation of many acids, by first converting into a lead salt, and
then submitting this salt to the action of sulphuretted hydrogen. For
example, lead formate with sulphuretted hydrogen gives formic acid.
Sulphuretted hydrogen in acting on a number of metallic acid substances
in solution or in an anhydrous state also forms corresponding sulphates:
(1) if it does not reduce the acid; (2) if the sulphur compound corresponding
with the anhydride of the acid be insoluble in water, the[213]
reaction proceeds in solutions; (3) if the sulphuretted hydrogen and the
acid taken do not come in contact with an alkali, on which they would
be able to act first; and (4) if the sulphur compound be not decomposed
by water. Thus solutions of arsenious acid give a precipitate of
arsenious sulphide, As2S3, with sulphuretted hydrogen. This reaction
proceeds not only in the presence of water, but also of acids, because
the latter do not decompose the resultant sulphur compounds. The
type of the decomposition is the same as with bases—that is, the
sulphur and oxygen change places: ROn+nH2S = RSn + nH2O. Some
sulphides corresponding with acid anhydrides are decomposed by
water, and therefore are not formed in the presence of water. Such,
for example, are the sulphides of phosphorus.[20]
The metallic sulphides corresponding with the metallic oxides
have either a feeble alkaline or a feeble acid character, according to
the character of the corresponding oxide, and therefore by combining[214]
together they are able to form saline substances—that is, salts in
which the oxygen is replaced by sulphur. Thus sulphuretted hydrogen
having the properties of a feeble acid[21] has, at the same time, the
properties of water, and forms the type of the sulphur derivatives,
which may also be formed by means of sulphuretted hydrogen, just as
the oxides may be formed by the aid of water. But as sulphuretted
hydrogen has acid properties, it combines more easily with the basic
metallic sulphides. Hence, for instance, there exists a compound
of sulphuretted hydrogen with potassium sulphide, potassium hydrosulphide,
2KHS = K2S + H2S, just as there are potassium hydroxides;
but there are scarcely any compounds of sulphuretted hydrogen with
the sulphides corresponding with acids. Thus the sulphides of the
metals may be regarded either as salts of sulphuretted hydrogen or as
oxides of the metals in which the oxygen is replaced by sulphur. In
general terms the sulphides exhibit the same degrees of difference with
respect to their solubility in water as do the oxides. Thus the oxides of
the alkali metals, and of some of the metals of the alkaline earths, are
soluble in water, whilst those of nearly all the other metals are insoluble.
The same may be said as to the sulphides; the sulphides of the metals
of the alkalis and certain of the alkaline earths are soluble in water,
whilst those of the other metals are insoluble. Those metals, like aluminium,
whose oxides—for example, Al2O3—have intermediate properties
and do not form compounds with feeble acids, at least in a
wet way, also do not form sulphides by this method, although these
may be obtained indirectly. And in general the sulphides of the metals
are easily formed in a wet way, and with particular ease if they are[215]
insoluble in water. In this case their salts enter into double decomposition
with sulphuretted hydrogen, or with soluble sulphides, and
give an insoluble sulphide—for instance, a salt of lead gives lead
sulphide with sulphuretted hydrogen. By the action of sulphuretted
hydrogen on a salt of a metal, a free acid must be formed besides the
metallic sulphide. Thus if a metal M be in a state of combination
MX2, then by the action of sulphuretted hydrogen there will be formed,
besides MS,[22] an acid 2HX. It is evident that sulphuretted hydrogen
will not precipitate an insoluble sulphide from the salts of those metals
whose sulphides react with free acid, such as zinc, iron, manganese, &c.
The reaction FeCl2 + H2S = FeS + 2HCl, and the like, do not take place
because the acid acts on the ferrous sulphide. Antimonious sulphide
is not acted on by dilute hydrochloric acid, but it is decomposed by
strong acid, and therefore in presence of an excess of hydrochloric acid
antimonious chloride does not entirely react with hydrogen sulphide,
whilst the reaction 2SbCl3 + 3H2S = Sb2S3 + 6HCl is a complete one
in a dilute solution and with a small quantity of acid. Those metallic
sulphides which are decomposed by acids may be obtained in a wet way
by the double decomposition of the salts of the metals, not with hydrogen
sulphide, but with soluble metallic sulphides, such as sulphide of
ammonium or of potassium, because then no free acid is formed, but a
salt of the metal (potassium or ammonium) which was taken as a
soluble sulphide. So, for example, FeCl2 + K2S = FeS + 2KCl.[23]
[216]
Metallic sulphides may be obtained by many other means besides
the action of sulphuretted hydrogen on salts and oxides, or by the
simple combination of metals with sulphur when heated or fused. Thus
they may also be formed by the reduction of sulphates by heating them
with charcoal or other means. Charcoal takes up the oxygen from
many sulphates, leaving corresponding sulphides. Thus sodium sulphate,
Na2SO4, when heated with charcoal, forms sodium sulphide,[217]
Na2S. Besides which metallic sulphides are also obtained by heating
metals or their oxides in the vapours of many sulphur compounds—for
example, in the vapour of carbon bisulphide, CS2, when the carbon takes
up the oxygen and the sulphur combines with the metal. The sulphides
formed in this manner are often crystalline, and often appear with
those properties and in that crystalline form in which they occur in
nature. Besides which we must mention that many of the sulphides
of the metals are oxidised in air at the ordinary, and especially at a
higher, temperature, forming either SO2 and the oxide of the metal or
sulphates. This oxidation proceeds with particular ease, even at the
ordinary temperature, when a metallic sulphide is precipitated from
its solutions, as a fine powder containing water. The sulphides of iron
and manganese, &c., are very easily oxidised in this manner. But if
these hydrates be ignited, they lose their water (the ignition must be
carried on in a stream of hydrogen to prevent their oxidation during
the process), become denser, and are no longer oxidised at the ordinary
temperature. Those sulphides whose corresponding sulphates are decomposed
by heat part with their sulphur in the form of sulphurous
anhydride when they are ignited in air, and the metal, as a rule,
remains behind as oxide. This is taken advantage of in the treatment
of sulphurous ores. The process is called roasting.
Hydrogen not only forms sulphuretted hydrogen with sulphur, but
it also combines with it in several other proportions, just as it combines
with oxygen, forming not only water but also hydrogen peroxide.
Moreover these polysulphides of hydrogen are also unstable, like
hydrogen peroxide, and are also obtained from the corresponding
polysulphides of the metals of the alkaline earths, just as hydrogen
peroxide is obtained from barium peroxide. Thus calcium forms not
only calcium sulphide, CaS, but also as bi-, tri-, and pentasulphide,
CaS5, and all these compounds are soluble in water. Sodium also combines
with sulphur in the same proportions, forming sulphides from
Na2S to Na2S5. If an acid be added to a solution of a polysulphide,
it gives sulphur, sulphuretted hydrogen, and a salt of the metal. For
instance, MS5, + 2HCl = MCl2 + H2S + 4S. If we reverse the operation,
and pour a solution of a polysulphide into an acid, sulphur is
not precipitated, but an oily liquid is formed which is heavier than
water and insoluble in it. This is the polysulphide of hydrogen:
MS5 + 2HCl = MCl2 + H2S5. As Rebs showed (1888), whatever
polysulphide be taken—of sodium, for instance—it always gives one
and the same hydrogen pentasulphide,[24] of specific gravity 1·71 (15°).[218]
It can only be preserved in the absence of water and at low temperatures,
and then not for long: for, especially in the presence of alkalis
and when slightly warmed, it splits up very easily into sulphuretted
hydrogen and sulphur.[25]
The soluble sulphides and polysulphides of the metals of the
alkalis and alkaline earths—for example, of ammonium,[26] potassium,[27][219]
and calcium,[28]—have the appearance and properties of salts,
just as the hydrated oxides have, whilst the sulphides of the metals of the[220]
[221]
higher groups resemble their oxides and have not at all the appearance
of salts, and this is more especially the case with regard to the crystalline
forms in which they frequently occur in nature.[29]
[222]
[223]
As the acids derived from chlorine, phosphorus, and carbon are
the oxidised hydrogen compounds of these elements, so also we can
form an idea of the acid hydrates of sulphur, or of the normal acids
of sulphur, by representing them as the oxidised products of sulphuretted
hydrogen—
| HCl |
H2S |
H3P |
H4C |
| HClO |
H2SO(?) |
H3PO(?) |
H4CO |
| HClO2 |
H2SO2(?) |
H3PO2 |
H4CO2 |
| HClO3 |
H2SO3 |
H3PO3 |
H4CO3 |
| HClO4 |
H2SO4 |
H3PO4 |
H4CO4[30] |
In the case of chlorine, if not all the hydrates, at all events salts of
all the normal hydrates are known, whilst in the case of sulphur only
the acids H2S, H2SO3 and H2SO4 are known. But, on the other hand,
the latter are obtained not only as hydrates but also as stable anhydrides,
SO2 and SO3, which are formed with the evolution of heat[224]
from sulphur and oxygen; 32 parts of sulphur in combining with
32 parts of oxygen—that is, in forming SO2—evolve 71,000 heat
units,[31] and if the oxidation proceeds to the formation of SO3, 103,000
heat units are evolved. These figures may be compared with those
which correspond with the passage of carbon into CO and CO2, when
29,000 and 97,000 units of heat are evolved. This determines the
stability of the higher oxides of sulphur, and also expresses the peculiarity
of sulphur as an element which, although an analogue of oxygen,
forms stable compounds with it, and thus fundamentally differs from
chlorine. The higher and lower oxides of chlorine are powerful oxidising
agents, whilst the higher oxide of sulphur, SO3, has but feeble
oxidising powers, and the lower oxide, SO2, frequently acts as a reducing
agent, and is formed by the direct combustion of sulphur, just
as carbonic anhydride, CO2, proceeds from the combustion of carbon.
In the combustion of sulphur, and also in the oxidation (roasting) of the
sulphides and polysulphides by their ignition in air, sulphurous oxide,
or sulphurous anhydride, or sulphur dioxide, SO2,[31 bis] is exclusively
formed. It is prepared on a large scale by burning sulphur or roasting
iron pyrites or other sulphides[32] for the manufacture of sulphuric
acid (Chapter VI.), and for direct application in the manufacture of
wine or for bleaching tissues and other purposes. In the latter instances
its application is based on the fact that sulphurous anhydride
acts on certain vegetable matters, and has the property of a reducing
and feeble acid.[32 bis]
[225]
In the laboratory—that is, on a small scale—sulphurous anhydride
is best prepared by deoxidising sulphuric acid by heating it with
charcoal, or copper, sulphur, mercury, &c. Charcoal produces this
decomposition of sulphuric acid at but moderately high temperatures;
it is itself converted into carbonic anhydride,[32 tri] and therefore when
sulphuric acid is heated with charcoal it evolves a mixture of sulphurous
and carbonic anhydrides: C + 2H2SO4 = CO2 + 2SO2 + 2H2O. The
metals which are unable to decompose water, and which do not, therefore,
expel hydrogen from sulphuric acid, are frequently capable of
decomposing sulphuric acid, with the evolution of sulphurous anhydride,
just as they decompose nitric acid, forming the lower oxides of nitrogen.
These metals are silver, mercury, copper, lead, and others. Thus, for
example, the action of copper on sulphuric acid may be expressed by
the following equation: Cu + 2H2SO4 = CuSO4 + SO2 + 2H2O. In
the laboratory this reaction is carried on in a flask with a gas-conducting
tube, and does not take place unless aided by heat.[33]
In its physical and chemical properties sulphurous anhydride
presents a great resemblance to carbonic anhydride. It is a heavy gas,
somewhat considerably soluble in water, very easily condensed into a
liquid; it forms normal and acid salts, does not evolve oxygen under
the direct action of heat,[34] although such metals as sodium and magnesium
burn in it, just as in carbonic anhydride. It has a suffocating
odour, which is well known owing to its being evolved when sulphur
or sulphur matches are burnt. In characterising the properties of
sulphurous anhydride, it is very important to remember (Chapter II.)
also that it is more easily liquefied (at -10°, or at 0° under two[226]
atmospheres pressure) than carbonic anhydride (thirty-six atmospheres
at 0°),[35] that it is more soluble than carbonic anhydride (Vol. I.
p. 79); at 0°, 100 vols. of water dissolve 180 vols. of carbonic
anhydride and 688 vols. of sulphuric anhydride), that the molecular
weight of SO2 = 64 and of CO2 = 44, and that the density of liquid sulphurous
anhydride at 0° = 1·43 (molecular volume = 45) and of carbonic
anhydride = 0·95 (molecular volume = 49). Although sulphur dioxide
is the anhydride of an acid, nevertheless, like carbonic anhydride, it does
not form any stable compounds with water, but gives a solution from
which it may be entirely expelled by the action of heat.[36] The acid
character of sulphurous anhydride is clearly expressed by the fact that
it is entirely absorbed by alkalis, with which it forms acid and normal
salts easily soluble in water. With salts of barium, calcium, and the
heavy metals, the normal salts of the alkalis, M2SO3, give precipitates
exactly like those formed by the carbonates. In general, the salts of
sulphurous acid are closely analogous to the corresponding carbonates.
Acid sodium sulphite, NaHSO3, may be obtained by passing sulphurous
anhydride into a solution of sodium hydroxide. It is also
formed by saturating a solution of sodium carbonate with the gas
(carbonic anhydride is then given off), and as the solubility of the acid
sulphite is much greater than that of the carbonate, a further quantity
of the latter may be dissolved after the passage of the sulphurous
anhydride, so that ultimately a very strong solution of the sulphite
may be formed in this manner, from which it may be obtained in a
crystalline form, either by cooling and evaporating (without heating,
for then the salt would give off sulphurous anhydride) or by adding
alcohol to the solution. When exposed to the air this salt loses
sulphurous anhydride and attracts oxygen, which converts it into
sodium sulphate. The acid sulphites of the alkali metals are able to
combine not only with oxygen, but also with many other substances—for
example, a solution of the sodium salt dissolves sulphur, forming
sodium thiosulphate, gives crystalline compounds with the aldehydes
and ketones, and dissolves many bases, converting them into double[227]
sulphites. Having the faculty of attracting or absorbing oxygen, acid
sodium sulphite is also able to absorb chlorine, and is therefore
employed, like sodium thiosulphate, for the removal of chloride (as an
antichlor), especially in the bleaching of fabrics, when it is necessary
to remove the last traces of the chlorine held in the tissues, which
might otherwise have an injurious effect on them. If a solution of an
alkali hydroxide be divided into two parts, and one half is saturated
with sulphurous anhydride, and then the other half added to it, a
normal salt will be obtained in the solution, having an alkaline reaction,
like a solution of sodium carbonate. The acid salt has a neutral
reaction.[36 bis] Like sodium carbonate, normal sodium sulphite has the
composition Na2SO3,10H2O, and its maximum solubility is at 33°—in
a word, it very closely resembles sodium carbonate. Although this
salt does not give off sulphurous anhydride from its solution, it is able,
like the acid salt, to absorb oxygen from the air, and is then converted
into sodium sulphate.[37]
Besides the acid character we must also point out the reducing
character of sulphurous anhydride. The reducing action of sulphurous
acid, its anhydride and salts, is due to their faculty of passing into
sulphuric acid and sulphates. The reducing action of the sulphites
is particularly energetic, so that they even convert nitric oxide into
nitrous oxide: K2SO3 + 2NO = K2SO4 + N2O. The salts of many of the
higher oxides are converted into those of the lower—for example, FeX3
into FeX2, CuX2 into CuX, HgX2 into HgX; thus 2FeX3 + SO2 + 2H2O
= 2FeX2 + H2SO4 + 2HX. In the presence of water, sulphurous
anhydride is oxidised by chlorine (SO2 + 2H2O + Cl2 = H2SO4 + 2HCl),
iodine, nitrous acid, hydrogen peroxide, hypochlorous acid, chloric acid,
and other oxygen compounds of the halogens, chromic, manganic, and
many other metallic acids and higher oxides, as well as all peroxides.
Free oxygen in the presence of spongy platinum is able to oxidise
sulphurous anhydride even in the absence of water, in which case
sulphuric anhydride SO3 is formed, so that the latter may be prepared
by passing a mixture of sulphurous anhydride and oxygen over
incandescent spongy platinum, or, as it is now prepared on a large scale
in chemical works, by passing this mixture over asbestos or pumice[228]
stone moistened with a solution of platinum salt and ignited. Sulphurous
anhydride is completely absorbed by certain higher oxides—for
instance, by barium peroxide and lead dioxide (PbO2 + SO2 = PbSO4).[38]
There are, however, cases where sulphurous anhydride acts as an
oxidising agent—that is, it is deoxidised in the presence of substances
which are capable of absorbing oxygen with still greater energy than
the sulphurous anhydride itself. This oxidising action proceeds with
the formation of sulphuretted hydrogen or of sulphides, while the
reducing agent is oxidised at the expense of the oxygen of the sulphurous
anhydride. In this respect, the action of stannous salts is
particularly remarkable. Stannous chloride, SnCl2, in an aqueous
solution gives a precipitate of stannic sulphide, SnS2, with sulphurous
anhydride—that is, the latter is deoxidised to sulphuretted hydrogen,
while SnX2 is oxidised into SnX4. A solution of sulphurous anhydride
has also an oxidising action on zinc. The zinc passes into solution, but no
hydrogen is evolved,[39] because a salt of hydrosulphurous acid, ZnS2O4,
is formed. The free acid is still less stable than the salt.
The faculty of sulphurous anhydride of combining with various
substances is evident from the above-cited reactions, where it combines
with hydrogen and with oxygen, and this faculty also appears in the[229]
fact that, like carbonic oxide, it combines with chlorine, forming a
chloranhydride of sulphuric acid, SO2Cl2, to which we shall afterwards
return. The same faculty for combination also appears in the salts of
sulphurous acid, in their liability to oxidation and in the exceedingly
characteristic formation of a peculiar series of salts obtained by Pelouze
and Frémy. At a temperature of -10° or below, nitric oxide NO is
absorbed by alkaline solutions of the alkali sulphites, forming a peculiar
series of nitrosulphates. At a higher temperature these salts are not
formed but the nitric oxide is reduced to nitrous oxide. But in the
cold the liquid saturated with nitric oxide after a certain time gives
prismatic crystals resembling those of nitre. The composition of the
potassium salt is K2SN2O3—that is, the salt contains the elements of
potassium sulphite and of nitric oxide.[40]
There are also several other substances, formed by the oxides of
nitrogen and sulphur, which belong to this class of complex and, under[230]
some circumstances, unstable compounds. In the manufacture of
sulphuric acid, both these classes of oxides come into contact with each
other in the lead chambers, and if there be insufficient water for the
formation of sulphuric acid they give crystalline compounds, termed
chamber crystals. As a rule, the composition of the crystals is expressed
by the formula NHSO3. This is a compound of the radicles
NO2 of nitric acid, and HSO3 of sulphuric acid, or nitro-sulphuric
acid, NO2.SHO3, if sulphuric acid be expressed as OH.SHO3 and nitric
by NO2.OH. The tabular crystals of this substance fuse at about 70°,
are formed both by the direct action of nitrous anhydride or nitric
peroxide (but not NO, which is not absorbed by sulphuric acid) on
sulphuric acid (Weltzien and others), and especially on sulphuric acid
containing an anhydride and the lower oxides of sulphur and nitric acid.[41]
Thiosulphuric acid, H2S2O3—that is, a compound of sulphurous acid
and sulphur—also belongs to the products of combination of sulphurous
acid. In the same way that sulphurous acid, H2SO3, gives H2SO4 with
oxygen, so it gives H2S2O3 with sulphur. In a free state it is very unstable,
and it is only known in the form of its salts proceeding from the
direct action of sulphur on the normal sulphites; if endeavours be
made to separate it in a free state, it immediately splits up into those
elements from which it might be formed—that is, into sulphur and
sulphurous acid. The most important of its salts is the sodium
thiosulphate (known as hyposulphite), Na2S2O3,5H2O, which occurs in
colourless crystals, and is unacted on by atmospheric oxygen either
when in a dry state or in solution. Many other salts of this acid are
easily formed by means of this salt,[41 bis] although this cannot be done[231]
with all bases, for such bases as alumina, ferric oxide, chromium oxide,
and others do not give compounds with thiosulphuric acid, just as they
do not form stable compounds with carbonic acid. Whenever these
salts might be formed, they (like the acid) split up into sulphurous acid
and sulphur, and furthermore the elements of thiosulphuric acid in
many cases act in a reducing manner, forming sulphuric acid and
taking up the oxygen from reducible oxides. Thus when treated with
a thiosulphate the soluble ferric salts give a precipitate of sulphur and
form ferrous salts. The thiosulphates of the metals of the alkalis are
obtained directly by boiling a solution of their sulphites with sulphur:
Na2SO3 + S = Na2S2O3. The same salts are formed by the action of
sulphurous anhydride on solutions of the sulphides; thus sodium
sulphide dissolved in water gives sulphur and sodium thiosulphate
when a stream of sulphurous anhydride is passed through it: 2Na2S
+ 3SO2 = 2Na2S2O3 + S. The polysulphides of the alkali metals
when left exposed to the air attract oxygen and also form thiosulphates.[42]
[232]
Although sulphur, oxidising at a high temperature, only forms a
small quantity of sulphuric anhydride, SO3, and nearly all passes into
sulphurous anhydride, still the latter may be converted into the higher
oxide, or sulphuric anhydride, SO3, by many methods. Sulphuric
anhydride is a solid crystalline substance at the ordinary temperature;
it is easily fusible (15°), and volatile (46°), and rapidly attracts moisture.
Although it is formed by the combination of sulphurous anhydride
with oxygen, it is capable of further combination. Thus it combines
with water, hydrochloric acid, ammonia, with many hydrocarbons,[233]
and even with sulphuric acid, boric and nitrous anhydrides, &c., and
also with bases which burn directly in its vapour, forming sulphates in
the presence of traces of moisture (see Chapter IX., Note 29). The
oxidation of sulphurous anhydride, SO2, into sulphuric anhydride, SO3,
is effected by passing a mixture of the former and dry oxygen or
air over incandescent spongy platinum. An increase of pressure
accelerates the reaction (Hanisch). If the product be passed into a
cold vessel, crystalline sulphuric anhydride is deposited upon the sides of
the vessel, but as it is difficult to avoid all traces of moisture it always
contains compounds of its hydrates: H2S2O7 and H2S4O13, whose
presence so modifies the properties of the anhydride (Weber) that
formerly two modifications of the anhydride were recognised. The
same sulphuric anhydride may be obtained from certain anhydrous
sulphates, or those which are almost so, which are decomposed by heat,
whilst an impure but perfectly anhydrous anhydride is formed by
distillation over phosphoric anhydride. For instance, acid sodium
sulphate, NaHSO4, and the pyro- or di-sulphate, Na2S2O7 (Chapter
XII.) formed from it, when ignited evolve sulphuric anhydride. Green
vitriol—that is, ferrous sulphate, FeSO4—belongs to the number of
those sulphates which easily give off sulphuric anhydride under the
action of heat. It contains water of crystallisation and parts with it
when it is heated, but the last equivalent of water is driven off with
difficulty, just as is the case with magnesium sulphate, MgSO47H2O;
however, when thoroughly heated, this evolution of sulphuric anhydride
does take place, although not completely, because at a high temperature a
portion of it is decomposed by the ferrous oxide (SO3 + 2FeO), which
is converted into ferric oxide, Fe2O3, and in consequence part of the
sulphuric anhydride is converted into sulphurous anhydride. Thus the
products of the decomposition of ferrous sulphate will be: ferric oxide,
Fe2O3, sulphurous anhydride, SO2, and sulphuric anhydride, SO3,
according to the equation: 2FeSO4 = Fe2O3 + SO2 + SO3. As water
still remains with the ferrous sulphate when it is heated, the result will
partially consist of the hydrate H2SO4, with anhydride, SO3, dissolved
in it. Sulphuric acid was for a long time prepared in this manner;
the process was formerly carried on on a large scale in the neighbourhood
of Nordhausen, and hence the sulphuric acid prepared from
ferrous sulphate is called fuming Nordhausen acid. At the present
time the fuming acid is prepared by passing the volatile products of the
decomposition of ferrous sulphate through strong sulphuric acid prepared
by the ordinary method. The sulphurous anhydride is insoluble
in it, but it absorbs the sulphuric anhydride. Sulphuric anhydride
may be prepared not only by igniting FeSO4 or sodium pyrosulphate,[234]
Na2S2O7 (the decomposition proceeds at 600°), but also by heating
a mixture of the latter and MgSO4 (Walters); in the former case a
stable double salt MgNa2(SO4)2 finally remains. It is also obtained
by the direct combination of SO2 and O under the action of spongy
platinum or asbestos coated with platinum black (C. Winkler's process).
Nordhausen sulphuric acid fumes in air, owing to its containing
and easily giving off sulphuric anhydride, and it is therefore also called
fuming sulphuric acid; these fumes are nothing but the vapour of
sulphuric anhydride combining with the moisture in the air and forming
non-volatile sulphuric acid (hydrate).[43]
Nordhausen sulphuric acid contains a peculiar compound of SO3
and H2SO4, or pyrosulphuric acid; an imperfect anhydride of sulphuric
acid, H2S2O7, analogous in composition with the salts Na2S2O7,
K2Cr2O7, and bearing the same relation to H2SO4 that pyrophosphoric
acid does to H3PO4. The bond holding the sulphuric acid and
anhydride together is unstable. This is obvious from the fact that the
anhydride may easily be separated from this compound, by the action of
heat. In order to obtain the definite compound, the Nordhausen acid
is cooled to 5°, or, better still, a portion of it is distilled until all the
anhydride and a certain amount of sulphuric acid have passed over into
the distillate, which will then solidify at the ordinary temperature,
because the compound H2SO4,SO3 fuses at 35°. Although this substance
reacts on water, bases, &c., like a mixture of SO3 + H2SO4, still[235]
since a definite compound, H2S2O7, exists in a free state and gives salts
and a chloranhydride, S2O5Cl2,[44] we must admit the existence of a
definite pyrosulphuric acid, like pyrophosphoric acid, only that the
latter has a far greater stability and is not even converted into a perfect
hydrate by water. Further, the salts M2S2O7 dissolved in water react
in the same manner as the acid salts MHSO4, whilst the imperfect
hydrates of phosphoric acid (for example, PHO3, H4P2O7) have independent
reactions even in an aqueous solution which distinguish them
and their salts from the perfect hydrates.
Fig. 87.—Concentration of sulphuric acid in glass retorts. The neck of each retort is attached to a
bent glass tube, whose vertical arm is lowered into a glass or earthenware vessel acting as a
receiver for the steam which comes over from the acid, as the former still contains a certain
amount of acid.
Sulphuric acid, H2SO4, is formed by the combination of its anhydride,
SO3, and water, with the evolution of a large amount of heat;
the reaction SO3 + H2O develops 21,300 heat units. The method of its
preparation on a large scale, and most of the methods employed
for its formation, are dependent on the oxidation of sulphurous
anhydride, and the formation of sulphuric anhydride, which forms
sulphuric acid under the action of water. The technical method of its
manufacture has been described in Chapter VI. The acid obtained
from the lead chambers contains a considerable amount of water, and
is also impure owing to the presence of oxides of nitrogen, lead compounds,
and certain impurities from the burnt sulphur which have
come over in a gaseous and vaporous state (for example, arsenic compounds).
For practical purposes, hardly any notice is taken of the
majority of these impurities, because they do not interfere with its general
qualities. Most frequently endeavours are only made to remove, as far
as possible, all the water which can be expelled.[45] That is, the object[236]
is to obtain the hydrate, H2SO4, from the dilute acid (60 per cent.),
and this is effected by evaporation by means of heat. Every given
mixture of water and sulphuric acid begins to part with a certain
amount of aqueous vapour when heated to a certain definite temperature.
At a low temperature either there is no evaporation of water, or there
can even be an absorption of moisture from the air. As the removal
of the water proceeds, the vapour tension of the residue decreases for
the same temperature, and therefore the more dilute the acid the lower
the temperature at which it gives up a portion of its water. In consequence
of this, the removal of water from dilute solutions of sulphuric
acid may be easily carried on (up to 75 p.c. H2SO4) in lead vessels,
because at low temperatures dilute sulphuric acid does not attack lead.
But as the acid becomes more concentrated the temperature at which
the water comes over becomes higher and higher, and then the acid[237]
begins to act on lead (with the evolution of sulphuretted hydrogen and
conversion of the lead into sulphate), and therefore lead vessels cannot
be employed for the complete removal of the water. For this purpose
the evaporation is generally carried on in glass or platinum retorts, like
those depicted in figs. 87 and 88.
Fig. 88.—Concentration of sulphuric acid in platinum retorts.
The concentration of sulphuric acid in glass retorts is not a continuous
process, and consists of heating the dilute 75 per cent. acid
until it ceases to give off aqueous vapour, and until acid containing
93–98 per cent. H2SO4 (66° Baumé) is obtained—and this takes place
when the temperature reaches 320° and the density of the residue
reaches 1·847 (66° Baumé).[46] The platinum vessels designed for the
continuous concentration of sulphuric acid consist of a still B, furnished
with a still head E, a connecting pipe E F, and a syphon tube H R,
which draws off the sulphuric acid concentrated in the boiler. A stream
of sulphuric acid previously concentrated in lead retorts to a density of
about 60° Baumé—i.e. to 75 per cent. or a sp. gr. of 1·7—runs continuously
into the retort through a syphon funnel E′. The apparatus is fed
from above, because the acid freshly supplied is lighter than that which
has already lost water, and also because the water is more easily
evaporated from the freshly supplied acid at the surface. The platinum[238]
retort is heated, and the steam coming off[47] is condensed in a worm
F G, whilst as fresh dilute acid is supplied to the boiler the acid already
concentrated is drawn off through the syphon tube H B, which is
furnished with a regulating cock by means of which the outflow of the
concentrated acid from the bottom of the retort can be so regulated
that it will always present one and the same specific gravity, corresponding
with the strength required. For this purpose the acid flowing
from the syphon is collected in a receiver R, in which a hydrometer,
indicating its density, floats; if its density be less than 66° Baumé,
the regulating cock is closed sufficiently to retard the outflow of sulphuric
acid, so as to lengthen the time of its evaporation in the retort.[48]
[239]
Strictly speaking, sulphuric acid is not volatile, and at its so-called
boiling-point it really decomposes into its anhydride and water; its
boiling-point (338°) being nothing else but its temperature of decomposition.
The products of this decomposition are substances boiling
much below the temperature of the decomposition of sulphuric acid.
This conclusion with regard to the process of the distillation of sulphuric
acid may be deduced from Bineau's observations on the vapour-density
of sulphuric acid. This density referred to hydrogen proved
to be half that which sulphuric acid should have according to its
molecular weight, H2SO4, in which case it should be 49, whilst the
observed density was equal to 24·5. Besides which, Marignac showed
that the first portions of the sulphuric acid distilling over contain less
of the elements of water than the portion which remains behind, or
which distils over towards the end. This is explained by the fact that
on distillation the sulphuric acid is decomposed, but a portion of the
water proceeding from its decomposition is retained by the remaining
mass of sulphuric acid, and therefore at first a mixture of sulphuric
acid and sulphuric anhydride—i.e. fuming sulphuric acid—is obtained
in the distillate. It is possible by repeating the distillation several times
and only collecting the first portions of the distillate, to obtain a
distinctly fuming acid. To obtain the definite hydrate H2SO4 it is
necessary to refrigerate a highly concentrated acid, of as great a purity
as possible, to which a small quantity of sulphuric anhydride has been
previously added. Sulphuric acid containing a small quantity (a fraction
of a per cent. by weight) of water only freezes at a very low temperature,
while the pure normal acid, H2SO4, solidifies when it is cooled below 0°,[240]
and therefore the normal acid first crystallises out from the concentrated
sulphuric acid. By repeating the refrigeration several times,
and pouring off the unsolidified portion, it is possible to obtain a pure
normal hydrate, H2SO4, which melts at 10°·4. Even at 40° it gives off
distinct fumes—that is, it begins to evolve sulphuric anhydride, which
volatilises, and therefore even in a dry atmosphere the hydrate H2SO4
becomes weaker, until it contains 1½ p.c. of water.[49]
In a concentrated form sulphuric acid is commercially known as oil
of vitriol, because for a long time it was obtained from green vitriol and
because it has an oily appearance and flows from one vessel into another
in a thick and somewhat sluggish stream, like the majority of oily
substances, and in this clearly differs from such liquids as water, spirit,
ether, and the like, which exhibit a far greater mobility. Among its
reactions the first to be remarked is its faculty for the formation of
many compounds. We already know that it combines with its anhydride,
and with the sulphates of the alkali metals; that it is soluble in
water, with which it forms more or less stable compounds. Sulphuric
acid, when mixed with water, develops a very considerable amount of
heat.[50]
Besides the normal hydrate H2SO4, another definite hydrate,[241]
H2SO4,H2O (84·48 per cent. of the normal hydrate, and 15·52 per cent.
of water) is known; it crystallises[50 bis] extremely easily in large six-sided[242]
prisms, which form above 0°—namely, at about +8°·5; when
heated to 210° it loses water.[51] If the hydrates H2SO4 and H2SO4,H2O
exist at low temperatures as definite crystalline compounds, and if
pyrosulphuric acid, H2SO4SO3, has the same property, and if they all
decompose with more or less ease on a rise of temperature, with the
disengagement of either SO3 or H2O, and in their ordinary form
present all the properties of simple solutions, it follows that between
sulphuric anhydride, SO3, and water, H2O, there exists a consecutive
series of homogeneous liquids or solutions, among which we
must distinguish definite compounds, and therefore it is quite justifiable
to look for other definite compounds between SO3 and H2O, beyond the
conditions for a change of state. In this respect we may be guided by
the variation of properties of any kind, proceeding concurrently with a
variation in the composition of a solution.
But only a few properties have been determined with sufficient
accuracy. In those properties which have been determined for many
solutions of sulphuric acid, it is actually seen that the above-mentioned
definite compounds are distinguished by distinctive marks of change.
As an example we may cite the variation of the specific gravity with a
variation of temperature (namely K = ds/dt, if s be the sp. gr. and t the
temperature). For the normal hydrate, H2SO4, this factor is easily
determined from the fact that—
s = 18528 - 10·65t + 0·013t2,
where s is the specific gravity at t (degrees Celsius) if the sp. gr. of
water at 4° = 10,000. Therefore K = 10·65 - 0·026t. This means that
at 0° the sp. gr. of the acid H2SO4 decreases by 10·65 for every rise of
a degree of temperature, at 10° by 10·39, at 20° by 10·13, at 30° by
9·87.[52] And for solutions containing slightly more anhydride than
the acid H2SO4 (i.e. for fuming sulphuric acid), as well as for solutions
containing more water, K is greater than for the acid H2SO4. Thus for
the solution SO3,2H2SO4, at 10° K = 11·0. On diluting the acid H2SO4[243]
K again increases until the formation of the solution H2SO4,H2O
(K = 11·1 at 10°), and then, on further dilution with water, it again
decreases. Consequently both hydrates H2SO4 and H2SO4,H2O are
here expressed by an alteration of the magnitude of K.

Fig. 89.—Diagram showing the variation of the factor (ds/dp) of the specific gravity of solutions of
sulphuric acid. The percentage quantities of the acid, H2SO4, are laid out on the axes of abscissæ.
The ordinates are the factors or rises in sp. gr. (water at 4 = 10,000) with the increase in the
quantity of H2SO4.
This shows that in liquid solutions it is possible by studying the
variation of their properties (without a change of physical state) to
recognise the presence or formation of definite hydrate compounds,
and therefore an exact investigation of the properties of solutions, of
their specific gravity for instance, should give direct indications of such
compounds.[53] The mean result of the most trustworthy determinations[244]
of this nature is given in the following tables. The first of these tables
gives the specific gravities (in vacuo, taking the sp. gr. of water at
4° = 1), at 0° (column 3), 15° (column 4), and 30° (column 5),[53 bis] for
solutions having the composition H2SO4 + nH2O (the value of n is
given in the first column), and containing p (column 2) per cent. (by
weight in vacuo) of H2SO4.[53 tri]
| n |
p |
0° |
15° |
30° |
| 100 |
5·16 |
1·0374 |
1·0341 |
1·0292 |
| 50 |
9·82 |
1·0717 |
1·0666 |
1·0603 |
| 25 |
17·88 |
1·1337 |
1·1257 |
1·1173 |
| 15 |
26·63 |
1·2040 |
1·1939 |
1·1837 |
| 10 |
35·25 |
1·2758 |
1·2649 |
1·2540 |
| 8 |
40·50 |
1·3110 |
1·2649 |
1·2998 |
| 6 |
47·57 |
1·3865 |
1·3748 |
1·3622 |
| 5 |
52·13 |
1·4301 |
1·4180 |
1·4062 |
| 4 |
57·65 |
1·4881 |
1·4755 |
1·4631 |
| 3 |
64·47 |
1·5635 |
1·5501 |
1·5370 |
| 2 |
73·13 |
1·6648 |
1·6500 |
1·6359 |
| 1 |
84·48 |
1·7940 |
1·7772 |
1·7608 |
| 0·5 |
91·59 |
1·8445 |
1·8284 |
1·8128 |
| H2SO4 |
100 |
1·8529 |
1·8372 |
1·8221 |
[245]
In the second table the first column gives the percentage amount p
(by weight) of H2SO4, the second column the weight in grams (S15)
of a litre of the solution at 15° (at 4° the weight of a litre of water
= 1,000 grams), the third column, the variation (dS/dt) of this weight for
a rise of 1°, the fourth column, the variation dS/dp of this weight (at
15°) for a rise of 1 per cent. of H2SO4, the fifth column, the difference
between the weight of a litre at 0° and 15° (S0 - S15), and the sixth
column, the difference between the weight of a litre at 15° and 30°
(S15 - S30).
| p |
S15 |
dS15/dt |
dS15/dp |
S0-S15 |
S15-S15 |
| 0 |
999·15 |
0·148 |
7·0 |
0·7 |
3·4 |
| 5 |
1033·0 |
0·27 |
6·8 |
3·1 |
5·0 |
| 10 |
1067·7 |
0·28 |
7·1 |
5·2 |
6·4 |
| 20 |
1141·9 |
0·58 |
7·7 |
8·6 |
8·9 |
| 30 |
1221·3 |
0·69 |
8·2 |
10·4 |
10·4 |
| 40 |
1306·6 |
0·75 |
8·8 |
11·3 |
11·2 |
| 50 |
1397·9 |
0·79 |
9·9 |
11·9 |
11·8 |
| 60 |
1501·2 |
0·86 |
10·8 |
13·0 |
12·7 |
| 70 |
1613·1 |
0·93 |
11·6 |
14·1 |
13·8 |
| 80 |
1731·4 |
1·04 |
11·0 |
15·8 |
15·4 |
| 90 |
1819·9 |
1·08 |
5·4 |
16·4 |
16·0 |
| 95 |
1837·6 |
1·03 |
+1·7 |
15·8 |
15·1 |
| 100 |
1837·2 |
1·03 |
-1·9[54] |
15·7 |
15·1 |
The figures in these tables give the means of finding the amount
of H2SO4 contained in a solution from its specific gravity,[55] and also
show that ‘special points’ in the lines of variation of the specific gravity
with the temperature and percentage composition correspond to certain
definite compounds of H2SO4 with OH2. This is best seen in the
variation of the factors (dS/dt and dS/dp) with the temperature and[246]
composition (columns 3, 4, second table). We have already mentioned
how the factor of temperature points to the existence of hydrates,
H2SO4 and H2SO4,H2O. As regards the factor dS/dp (giving the
increase of sp. gr. with an increase of 1 per cent. H2SO4) the following
are the three most salient points: (1) In passing from 98 per cent. to
100 per cent. the factor is negative, and at 100 per cent. about -0·0019
(i.e. at 99 per cent. the sp. gr. is about 1·8391, and at 100 per cent.
about 1·8372, at 15°, the amount of H2SO4 has increased whilst the
sp. gr. has decreased), but as soon as a certain amount of SO3 is
added to the definite compound H2SO4 (and ‘fuming’ acid formed)
the specific gravity rises (for example, for H2SO4 0·136 SO3 the sp. gr.
at 15° = 1·866), that is the factor becomes positive (and, in fact, greater
by +0·01), so that the formation of the definite hydrate H2SO4 is
accompanied by a distinct and considerable break in the continuity
of the factor[55 bis]; (2) The factor (dS/dp) in increasing in its passage
from dilute to concentrated solutions, attains a maximum value (at 15°
about 0·012) about H2SO42H2O, i.e. at about the hydrate corresponding
to the form SX6; proper to the compounds of sulphur, for S(OH)6
= H2SO42H2O; the same hydrate corresponds to the composition of
gypsum CaSO42H2O, and to it also corresponds the greatest contraction
and rise of temperature in mixing H2SO4 with H2O (see Chapter I.,
Note 28); (3) The variation of the factor (dS/dp) under certain variations
in the composition proceeds so uniformly and regularly, and is so
different from the variation given under other proportions of H2SO4 and
H2O, that the sum of the variations of dS/dp is expressed by a series
of straight lines, if the values of p be laid along the axis of abscissæ
and those of dS/dp along the ordinates.[56] Thus, for instance, for 15°, at[247]
10 per cent. dS/dp = 0·0071, at 20 per cent. = 0·0077, at 30 per cent.
= 0·0082, at 40 per cent. = 0·0088, that is, for each 10 per cent. the
factor increases by about 0·0006 for the whole of the above range, but
beyond this it becomes larger, and then, after passing H2SO42H2O,
it begins to fall rapidly. Such changes in the variation of the factor
take place apparently about definite hydrates,[56 bis] and especially
about H2SO44H2O, H2SO42H2O and H2SO4H2O. All this indicating
as it does the special chemical affinity of sulphuric acid for water,
although of no small significance for comprehending the nature of solutions
(see Chapter I. and Chapter VII.), contains many special points
which require detailed investigation, the chief difficulty being that it
requires great accuracy in a large number of experimental data.
The great affinity of sulphuric acid for water is also seen from[248]
the fact that when the strong acid acts on the majority of organic
substances containing hydrogen and oxygen (especially on heating) it
very frequently takes up these elements in the form of water. Thus
strong sulphuric acid acting on alcohol, C2H6O, removes the elements
of water from it, and converts it into olefiant gas, C2H4. It acts in a
similar manner on wood and other vegetable tissues, which it chars.
If a piece of wood be immersed in strong sulphuric acid it turns black.
This is owing to the fact that the wood contains carbohydrates which
give up hydrogen and oxygen as water to the sulphuric acid, leaving
charcoal, or a black mass very rich in it. For example, cellulose,
C6H10O5, acts in this manner.[57]
We have already had frequent occasion to notice the very
energetic acid properties of sulphuric acid, and therefore we will now
only consider a few of their aspects. First of all we must remember
that, with calcium, strontium, and especially with barium and lead,
sulphuric acid forms very slightly soluble salts, whilst with the
majority of other metals it gives more easily soluble salts, which in the
majority of cases are able, like sulphuric acid itself, to combine with
water to form crystallo-hydrates. Normal sulphuric acid, containing
two atoms of hydrogen in its molecule, is able for this reason alone
to form two classes of salts, normal and acid, which it does with great
facility with the alkali metals. The metals of the alkaline earths
and the majority of other metals, if they do form acid sulphates, do so
under exceptional conditions (with an excess of strong sulphuric acid),
and these salts when formed are decomposable by water—that is,
although having a certain degree of physical stability they have no
chemical stability. Besides the acid salts RHSO4, sulphuric acid also
gives other forms of acid salts. An entire series of salts having the
composition RHSO4,H2SO4, or for bivalent metals RSO4,3H2SO4,[58]
has been prepared. Such salts have been obtained for potassium,
sodium, nickel, calcium, silver, magnesium, manganese. They are prepared[249]
by dissolving the sulphates in an excess of sulphuric acid and
heating the solution until the excess of sulphuric acid is driven off;
on cooling, the mass solidifies to a crystalline salt. Besides which,
Rose obtained a salt having the composition Na2SO4,NaHSO4, and if
HNaSO4 be heated it easily forms a salt Na2S2O7 = Na2SO4,SO3;
hence it is clear that sulphuric anhydride combines with various proportions
of bases, just as it combines with various proportions of
water.
We have already learned that sulphuric acid displaces the acid from
the salts of nitric, carbonic, and many other volatile acids. Berthollet's
laws (Chapter X.) explain this by the small volatility of sulphuric
acid; and, indeed, in an aqueous solution sulphuric acid displaces the
much less soluble boric acid from its compounds—for instance, from
borax, and it also displaces silica from its compounds with bases; but
both boric anhydride and silica, when fused with sulphates, decompose
them, displacing sulphuric anhydride, SO3, because they are less
volatile than sulphuric anhydride. It is also well known that with
metals, sulphuric acid forms salts giving off hydrogen (Fe, Zn, &c.), or
sulphur dioxide (Cu, Hg, &c.).[58 bis]
The reactions of sulphuric acid with respect to organic substances
are generally determined by its acid character, when the direct extraction
of water, or oxidation at the expense of the oxygen of the
sulphuric acid,[59] or disintegration does not take place. Thus the
majority of the saturated hydrocarbons, CnH2m, form with sulphuric
acid a special class of sulphonic acids, CnH2m-1(HSO3); for example,[250]
benzene, C6H6, forms benzenesulphonic acid, C6H5.SO3H, water being
separated, for the formation of which oxygen is taken up from the
sulphuric acid, for the product contains less oxygen than the sulphuric
acid. It is evident from the existence of these acids that the hydrogen in
organic compounds is replaceable by the group SO3H, just as it may be
replaced by the radicles Cl, NO2, CO2H and others. As the radicle
of sulphuric acid or sulphoxyl, SO2OH or SHO3, contains, like carboxyl
(Vol. I., p. 395), one hydrogen (hydroxyl) of sulphuric acid, the
resultant substances are acids whose basicity is equal to the number
of hydrogens replaced by sulphoxyl. Since also sulphoxyl takes the
place of hydrogen, and itself contains hydrogen, the sulpho-acids are
equal to a hydrocarbon + SO3, just as every organic (carboxylic) acid
is equal to a hydrocarbon + CO2. Moreover, here this relation corresponds
with actual fact, because many sulphonic acids are obtained
by the direct combination of sulphuric anhydride: C6H5,(SO3H)
= C6H6 + SO3. The sulphonic acids give soluble barium salts, and
are therefore easily distinguished from sulphuric acid. They are
soluble in water, are not volatile, and when distilled give sulphurous
anhydride (whilst the hydroxyl previously in combination with the
sulphurous anhydride remains in the hydrocarbon group; thus phenol,
C6H5.OH, is obtained from benzenesulphonic acid), and they are very
energetic, because the hydrogen acting in them is of the same nature as
in sulphuric acid itself.[60]
Sulphuric acid, as containing a large proportion of oxygen, is a[251]
substance which frequently acts as an oxidising agent: in which case
it is deoxidised, forming sulphurous anhydride and water (or even,
although more rarely, sulphuretted hydrogen and sulphur). Sulphuric
acid acts in this manner on charcoal, copper, mercury, silver, organic
and other substances, which are unable to evolve hydrogen from it
directly, as we saw in describing sulphurous anhydride.
Although the hydrate of a higher saline form of oxidation (Chapter
XV.), sulphuric anhydride is capable of further oxidation, and forms a
kind of peroxide, just as hydrogen gives hydrogen peroxide in addition
to water, or as sodium and potassium, besides the oxides Na2O and
K2O, give their peroxides, compounds which are in a chemical sense
unstable, powerfully oxidising, and not directly able to enter into saline
combinations. If the oxides of potassium, barium, &c., be compared
to water, then their peroxides must in like manner correspond to
hydrogen peroxide,[61] not only because the oxygen contained in them
is very mobile and easily liberated, and because their reactions are
similar, but also because they can be mutually transformed into each
other, and are able to form compounds with each other, with bases
and with water, and indeed form a kind of peroxide salts.[62] This
is also the character of persulphuric acid, discovered in 1878 by
Berthelot, and its corresponding anhydride or peroxide of sulphur S2O7.
It is formed from 2SO3 + O with the absorption of heat (-27
thousand heat units), like ozone from O2 + O (-29 thousand units of
heat), or hydrogen peroxide from H2O + O (-21 thousand heat units).
Peroxide of sulphur is produced by the action of a silent discharge
upon a mixture of oxygen and sulphurous anhydride.[63] With water[252]
S2O7 gives persulphuric acid, H2S2O8. The latter is obtained
more simply by mixing strong sulphuric acid (not weaker than
H2SO4,2H2O) directly with hydrogen peroxide, or by the action of a
galvanic current on sulphuric acid mixed with a certain amount of water,
and cooled, the electrodes being platinum wires, when persulphuric acid
naturally appears at the positive pole.[64] When an acid of the strength
H2SO4,6H2O is taken, at first the hydrate of the sulphuric peroxide,
S2O7,H2O only is formed; but when the concentration about
the positive pole reaches H2SO4,3H2O, a mixture of hydrogen peroxide
and the hydrate of sulphuric peroxide begins to be formed. Dilute
solutions of sulphuric peroxide can be kept better than more concentrated
solutions, but the latter may be obtained containing as
much as 123 grams of the peroxide to a litre. It is a very instructive
fact that hydrogen peroxide is always formed when strong solutions of
persulphuric acid break up on keeping. So that the bond between the
two peroxides is established both by analysis and synthesis: hydrogen
peroxide is able to produce S2H2O8, and the latter to produce hydrogen
peroxide. A mixture of sulphuric peroxide with sulphuric acid or
water is immediately decomposed, with the evolution of oxygen, either
when heated or under the action of spongy platinum. The same thing[253]
takes place with a solution of baryta, although at first no precipitate is
formed and the decomposition of the barium salt, BaS2O8, with the formation
of BaSO4, only proceeds slowly, so that the solution may be
filtered (the barium salt of persulphuric acid is soluble in water). Mercury,
ferrous oxide, and the stannous salts, are oxidised by S2H2O8.
These are all distinct signs of true peroxides. The same common properties
(capacity for oxidising, property of forming peroxide of hydrogen,
&c.) are possessed by the alkali salts of persulphuric acid, which are
obtained by the action of an electric current upon certain sulphates,
for instance ammonium or potassium sulphate. The ammonium salt
of persulphuric acid, (NH4)2S2O8, is especially easily formed by this
means, and is now prepared on a large scale and used (like Na2O2 and
H2O2) for bleaching tissues and fibres.[65]
[254]
In order to understand the relation of sulphuric peroxide to sulphuric
acid we must first remark that hydrogen peroxide is to be considered,
in accordance with the law of substitution, as water, H(OH), in which
H is replaced by (OH). Now the relation of H2S2O8 to H2SO4 is
exactly similar. The radicle of sulphuric acid, equivalent to hydrogen,
is HSO4;[65 bis] it corresponds with the (OH) of water, and therefore
sulphuric acid, H(SHO4), gives (SHO4)2 or S2H2O8, in exactly the same
manner as water gives (HO)2—i.e. H2O2.[66]
The largest part of the sulphuric acid made is used for reacting
on sodium chloride in the manufacture of sodium carbonate; for the
manufacture of the volatile acids, like nitric, hydrochloric, &c., from
their corresponding salts; for the preparation of ammonium sulphate,
alums, vitriols (copper and iron), artificial manures, superphosphate
(Chapter XIX., Note 18) and other salts of sulphuric acid; in the treatment
of bone ash for the preparation of phosphorus, and for the solution
of metals—for example, of silver in its separation from gold—for[255]
cleaning metals from rust, &c. A large amount of oil of vitriol is also
used in treatment of organic substances; it is used for the extraction
of stearin, or stearic acid, from tallow, for refining petroleum and
various vegetable oils, in the preparation of nitro-glycerine (Chapter VI.,
Notes 37 and 37 bis), for dissolving indigo and other colouring matters,
for the conversion of paper into vegetable parchment, for the preparation
of ether from alcohol, for the preparation of various artificial scents
from fusel oil, for the preparation of vegetable acids, such as oxalic,
tartaric, citric, for the conversion of non-fermentable starchy substances
into fermentable glucose, and in a number of other processes. It
would be difficult to find another artificially-prepared substance
which is so frequently applied in the arts as sulphuric acid. Where
there are not works for its manufacture, the economical production of
many other substances of great technical importance is impossible.
In those localities which have arrived at a high technical activity
the amount of sulphuric acid consumed is proportionally large;
sulphuric acid, sodium carbonate, and lime are the most important of
the artificially-prepared agents employed in factories.
Besides the normal acids of sulphur, H2SO3, H2SO3S, and H2SO4,
corresponding with sulphuretted hydrogen, H2S, in the same way
that the oxy-acids of chlorine correspond with hydrochloric acid, HCl,
there exists a peculiar series of acids which are termed thionic acids.
Their general composition is SnH2O6, where n varies from 2 to 5. If
n = 2, the acid is called dithionic acid. The others are distinguished
as trithionic, tetrathionic, and pentathionic acids. Their composition,
existence, and reactions are very easily understood if they be referred
to the class of the sulphonic acids—that is, if their relation to
sulphuric acid be expressed in just the same manner as the relation of
the organic acids to carbonic acid. The organic acids, as we saw
(Chapter IX.), proceed from the hydrocarbons by the substitution of
their hydrogen by carboxyl—that is, by the radicle of carbonic acid,
CH2O3 - HO = CHO2. The formation of the acids of sulphur by
means of sulphoxyl may be represented in the same manner, HSO3
= H2SO4 - HO. Therefore to hydrogen H2, there should correspond the
acids H.SHO3, sulphurous, and SHO3.SHO3 = S2H2O6, or dithionic;
to SH2 there should correspond the acids SH(SHO3) = H2S2O3 (thiosulphuric),
and S(SHO3)2 = H2S3O6 (trithionic); to S2H2 the acids
S2H(SHO3) = H2S3O2 (unknown), and S2(SHO3)2 = H2S4O6 (tetrathionic);
to S3H2 the acids S3H(SHO3) and S3(SHO3)2 = H2S5O6
(pentathionic). We know that iodine reacts directly with the hydrogen
of sulphuretted hydrogen and combines with it, and if thiosulphuric
acid contains the radicle of sulphuretted hydrogen (or hydrogen united[256]
with sulphur) of the same nature as in sulphuretted hydrogen, it is not
surprising that iodine reacts with sodium thiosulphate and forms sodium
tetrathionate. Thus, thiosulphuric acid, HS(SHO3), when deprived
of H, gives a radicle which immediately combines with another similar
radicle, forming the tetrathionate S2(SO2HO)2. On this view[67] of
the structure of the thionic acids and salts, it is also clear how all the
thionic acids, like thiosulphuric acid, easily give sulphur and sulphides,
with the exception only of dithionic acid, H2S2O6, which, judging
from the above, stands apart from the series of the other thionic
acids. Dithionic acid stands in the same relation to sulphuric acid as
oxalic acid does to carbonic acid. Oxalic acid is dicarboxyl, (CHO2)2
= C2H2O4, and so also dithionic acid is disulphoxyl, (SHO3)2 = S2H2O6.
Oxalic acid when ignited decomposes into carbonic anhydride and
carbonic oxide, CO, and dithionic acid when heated decomposes into
sulphuric anhydride and sulphurous anhydride, SO2, and SO2 stands
in the same relation to SO3 as CO to CO2. This also explains the
peculiarity of the calcium, barium, and lead, &c. salts of the thionic acids
being easily soluble (although the corresponding salts of H2SO3, H2SO4,
and H2S dissolve with difficulty), because the former are similar to
the salts of the sulphonic acids, which are also soluble in water.
Thus the thionic acids are disulphonic acids, just as many dicarboxylic
acids are known—for example, CH2(CO2H)2, C6H4(CO2H)2.[68]
[257]
[258]
[259]
Sulphur exhibits an acid character, not only in its compounds with
hydrogen and oxygen, but also in those with other elements. The
compound of sulphur and carbon has been particularly well investigated.
It presents a great analogy to carbonic anhydride, both in its elementary
composition and chemical character. This substance is the so-called
carbon bisulphide, CS2, and corresponds with CO2.
The first endeavours to obtain a compound of sulphur with carbon
were unsuccessful, for although sulphur does combine directly with
carbon, yet the formation of this compound requires distinctly
definite conditions. If sulphur be mixed with charcoal and heated, it
is simply driven off from the latter, and not the smallest trace of
carbon bisulphide is obtained. The formation of this compound
requires that the charcoal should be first heated to a red heat, but not
above, and then either the vapour of sulphur passed over it or lumps
of sulphur thrown on to the red-hot charcoal, but in small quantities,
so as not to lower the temperature of the latter. If the charcoal be
heated to a white heat, the amount of carbon bisulphide formed is less.
This depends, in the first place, on the carbon bisulphide dissociating
at a high temperature.[69] In the second place, Favre and Silberman
showed that in the combustion of one gram of carbon bisulphide (the
products will be CO2 + 2SO2) 3,400 heat units are evolved—that is,
the combustion of a molecular quantity of carbon bisulphide evolves
258,400 heat units (according to Berthelot, 246,000). From a molecule
of carbon bisulphide in grams we may obtain 12 grams of carbon,
whose combustion evolves 96,000 heat units, and 64 grams of sulphur,
evolving by combustion (into SO2) 140,800 heat units. Hence we see
that the component elements separately evolve less heat by their combustion
(237,000 heat units) than carbon bisulphide itself—that is,[260]
that heat should be evolved (at the ordinary temperature) and not
absorbed in its decomposition, and therefore that the formation of carbon
bisulphide from charcoal and sulphur is in all probability accompanied
by an absorption of heat.[70] It is therefore not surprising that,
like other compounds produced with an absorption of heat (ozone,
nitrous oxide, hydrogen peroxide, &c.), carbon bisulphide is unstable
and easily converted into the original substances from which it is
obtained. And indeed if the vapour of carbon bisulphide be passed
through a red-hot tube, it is decomposed—that is, it dissociates—into
sulphur and carbon. And this takes place at the temperature at which
this substance is formed, just as water decomposes into hydrogen and
oxygen at the temperature of its formation. In this absorption of heat
in the formation of carbon bisulphide is explained the facility with which
it suffers reactions of decomposition, which we shall see in the sequel,
and its main difference from the closely analogous carbonic anhydride.
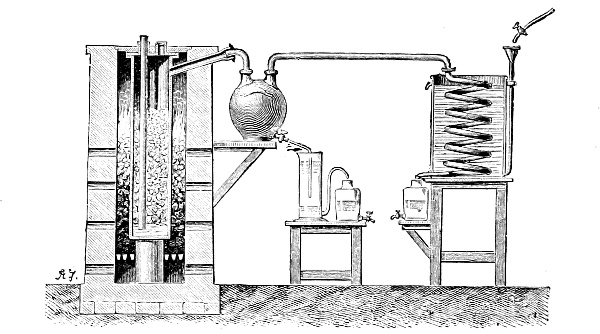
Fig. 90.—Apparatus for the manufacture of carbon bisulphide.
[261]
In the laboratory carbon bisulphide is prepared as follows: A
porcelain tube is luted into a furnace in an inclined position, the upper
extremity of the tube being closed by a cork, and the lower end
connected with a condenser. The tube contains charcoal, which is
raised to a red heat, and then pieces of sulphur are placed in the upper
end. The sulphur melts, and its vapour comes into contact with the
red-hot charcoal, when combination takes place; the vapours condense
in the condenser, carbon bisulphide being a liquid boiling at
48°. On a large scale the apparatus depicted in fig. 90 is employed.
A cast-iron cylinder rests on a stand in a furnace. Wood charcoal is
charged into the cylinder through the upper tube closed by a clay
stopper, whilst the sulphur is introduced through a tube reaching to
the bottom of the cylinder. Pieces of sulphur thrown into this tube
fall on to the bottom of the cylinder, and are converted into vapour,
which passes through the entire layer of charcoal in the cylinder. The
vapour of carbon bisulphide thus formed passes through the exit tube
first into a Woulfe's bottle (where the sulphur which has not entered
into the reaction is condensed), and then into a strongly-cooled condenser
or worm.[71]
Pure carbon bisulphide is a colourless liquid, which refracts light
strongly, and has a pure ethereal smell; at 0° its specific gravity is 1·293,
and at 15° 1·271. If kept for a long time it seems to undergo a change,
especially when it is kept under water, in which it is insoluble. It
boils at 48°, and the tension of its vapour is so great that it evaporates
very easily, producing cold,[72] and therefore it has to be kept in well-stoppered
vessels; it is generally kept under a layer of water, which
hinders its evaporation and does not dissolve it.[73]
[262]
Carbon bisulphide enters into many combinations, which are frequently
closely analogous to the compounds of carbonic anhydride. In
this respect it is a thio-anhydride—i.e. it has the character of the acid
anhydrides,[73 bis] like carbonic anhydride, with the difference that the
oxygen of the latter is replaced by sulphur. By thio-compounds in
general are understood those compounds of sulphur which differ from
the compounds of oxygen as carbon bisulphide does from carbonic
anhydride—that is, which correspond with the oxygen compounds, but
with substitution of sulphur for oxygen. Thus thiosulphuric acid is
monothiosulphuric acid—that is, sulphuric acid in which one atom
of sulphur replaces one atom of oxygen. With the sulphides of the
alkalis and alkaline earths, it forms saline substances corresponding
with the carbonates, and these compounds may be termed thiocarbonates.
For example, the composition of the sodium salt Na2CS3 is
exactly like that of sodium carbonate. They are formed by the direct
solution of carbon bisulphide in aqueous solutions of the sulphides; but
they are difficult to obtain in a crystalline form, because they are easily
decomposable. When the solutions of these salts are highly concentrated
they begin to decompose, with the evolution of sulphuretted
hydrogen and the formation of a carbonate, water taking part in the
reaction—for example, K2CS3 + 3H2O = K2CO3 + 3H2S.[74]
[263]
A remarkable example[74 bis] of the thio-compounds is found in thiocyanic
acid—i.e. cyanic acid in which the oxygen is replaced by
sulphur, HCNS. We know (Chapter IX.) that with oxygen the
cyanides of the alkaline metals RCN give cyanates RCNO; but they[264]
also combine with sulphur, and therefore if yellow prussiate of potash
be treated as in the preparation of potassium cyanide, and sulphur be
added to the mass, potassium thiocyanate, KNCS, is obtained in
solution. This salt is much more stable than potassium cyanate; it
dissolves without change in water and alcohol, forming colourless
solutions from which it easily crystallises on evaporation. It may be
kept exposed to air even when in solution; in dissolving in water it
absorbs a considerable amount of heat, and forms a starting-point for
the preparation of all the thiocyanates, RCNS, and organic compounds
in which the metals are replaced by hydrocarbon groups. Such, for
example, is volatile mustard oil, C3H5CSN (allyl thiocyanate),[75] which
gives to mustard its caustic properties. With ferric salts the thiocyanates
give an exceedingly brilliant red coloration, which serves for detecting
the smallest traces of ferric salts in solution. Thiocyanic acid, HCNS,
may be obtained by a method of double decomposition, by distilling
potassium thiocyanate with dilute sulphuric acid. It is a volatile
colourless liquid, having a smell recalling that of vinegar, is soluble in
water, and may be kept in solution without change.[75 bis]
The sulphur compounds of chlorine Cl2S and Cl2S2 may be regarded
on the one hand as products of the metalepsis of the sulphides of
hydrogen, H2S and H2S2; and on the other hand of the oxygen compounds
of chlorine, because chloride of sulphur, Cl2S, resembles chlorine
oxide, Cl2O, whilst Cl2S2 corresponds with the higher oxide of chlorine;
or thirdly, we may see in these compounds the type of the acid chloranhydrides,
because they are all decomposed by water, forming hydrochloric[265]
acid, and sulphur tetrachloride, SCl4, is decomposed with the formation
of sulphurous anhydride.[76]
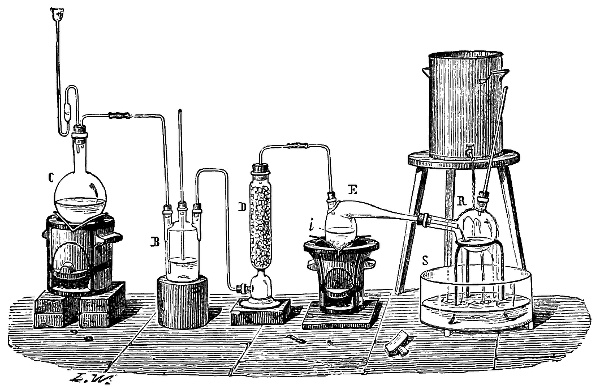
Fig. 91.—Apparatus for the preparation of sulphur chloride, and similar volatile compounds
prepared by combustion in a stream of chlorine.
The compounds of sulphur with chlorine are prepared in the
apparatus depicted in fig. 91. As sulphur chloride is decomposed by
water, the chlorine evolved in the flask C must be dried before coming
into contact with the sulphur. It is therefore first passed through a
Woulfe's bottle, B, containing sulphuric acid, and then through the
cylinder D containing pumice stone moistened with sulphuric acid, and
then led into the retort E, in which the sulphur is heated. The compound
which is formed distils over into the receiver R. A certain
amount of sulphur passes over with the sulphur chloride, but if the
resultant distillate be re-saturated with chlorine and distilled no free
sulphur remains, the boiling-point rises to 144°, and pure sulphur
chloride, S2Cl2, is obtained. It has this formula because its vapour
density referred to hydrogen is 68. It is also obtained by heating
certain metallic chlorides (stannous, mercuric) with sulphur; both the[266]
metal and chlorine then combine with the sulphur. Sulphur chloride
is a yellowish-brown liquid, which boils at 144°, and has a specific gravity
of 1·70 at 0°. It fumes strongly in the air, reacting on the moisture
contained therein, and has a heavy chloranhydrous odour. It dissolves
sulphur, is miscible with carbon bisulphide, and falls to the bottom of a
vessel containing water, by which it is decomposed, forming sulphurous
anhydride and hydrochloric acid; but it first forms various lower
stages of oxidation of sulphur, because the addition of silver nitrate to
the solution gives a black precipitate. With hydrogen sulphide it gives
sulphur and hydrochloric acid, and it reacts directly with metals—especially
arsenic, antimony, and tin—forming sulphides and chlorides.
In the cold, it absorbs chlorine and gives sulphur dichloride, SCl2. The
entire conversion into this substance requires the prolonged passage of
dry chlorine through sulphur chloride surrounded by a freezing mixture.
The distillation of the dichloride must be conducted in a stream of
chlorine, as otherwise it partially decomposes into sulphur chloride and[267]
chlorine. Pure sulphur dichloride is a reddish-brown liquid, which
resembles the lower chloride in many respects; its specific gravity is
1·62; its odour is more suffocating than that of sulphur chloride; it
volatilises at 64°.[77]
Thionyl chloride, SOCl2, may be regarded as oxidised sulphur
dichloride; it corresponds with sulphur chloride, S2Cl2, in which one
atom of sulphur is replaced by oxygen. At the same time it is chlorine
oxide (hypochlorous anhydride, Cl2O) combined with sulphur, and also
the chloranhydride of sulphurous acid—that is, SO(HO)2, in which the
two hydroxyl groups are replaced by two atoms of chlorine, or sulphurous
anhydride, SO2, in which one atom of oxygen is replaced by two
atoms of chlorine. All these representations are confirmed by reactions
of formation, or decompositions; they all agree with our notions of the
other compounds of sulphur, oxygen, and chlorine; hence these definitions
are not contradictory to each other. Thus, for instance, thionyl
chloride was first obtained by Schiff, by the action of dry sulphurous
anhydride on phosphorus pentachloride. On distilling the resultant
liquid, thionyl chloride comes over first at 80°, and on continuing
the distillation phosphorus oxychloride distils over at above 100°,
PCl5 + SO2 = POCl3 + SOCl2. This mode of preparation is direct evidence
of the oxychloride character of SOCl2. Würtz obtained the same
substance by passing a stream of chlorine oxide through a cold solution
of sulphur in sulphur chloride; the chlorine oxide then combined directly
with the sulphur, S + Cl2O = SOCl2, whilst the sulphur chloride remained
unchanged (sulphur cannot be combined directly with chlorine oxide, as
an explosion takes place). Thionyl chloride is a colourless liquid, with
a suffocating acrid smell; it has a specific gravity at 0° of 1·675, and boils[268]
at 78°. It sinks in water, by which it is immediately decomposed, like
all chloranhydrides—for example, like carbonyl chloride, which corresponds
with it: SOCl2 + H2O = SO2 + 2HCl.[77 bis]
Normal sulphuric acid has two corresponding chloranhydrides; the
first, SO2(OH)Cl, is sulphuric acid, SO2(HO)2, in which one equivalent
of HO is replaced by chlorine; the second has the composition SO2Cl2—that
is, two HO groups are substituted by two of chlorine. The second
chloranhydride, or the compound SO2Cl2, is called sulphuryl chloride,
and the first chloranhydride, SO2HOCl, may be called chlorosulphonic
acid, because it is really an acid; it still retains one hydroxyl of sulphuric
acid, and its corresponding salts are known. Thus, potassium
chloride absorbs the vapour of sulphuric anhydride, forming a salt,
SO3KCl, corresponding with SO3HCl as acid. In acting on sodium
chloride it forms hydrochloric acid and the salt NaSO3Cl. This first
chloranhydride of sulphuric acid, SO2HOCl, discovered by Williamson,
is obtained either by the action of phosphorus pentachloride on sulphuric
acid (PCl5 + H2SO4 = POCl3 + HCl + HSO3Cl), or directly by the action
of dry hydrochloric acid on sulphuric anhydride, SO3 + HCl = HSO3Cl.
The most easy and rapid method of its formation is by direct saturation
of cold Nordhausen acid with dry hydrochloric acid gas (SO3 + HCl
= HSO3Cl), and distillation of the resultant solution; the distillate
then contains HSO3Cl. It is a colourless fuming liquid, having an acrid
odour; it boils at 153° (according to my determination, confirmed by
Konovaloff), and its specific gravity at 19° is 1·776. It is immediately
decomposed by water, forming hydrochloric and sulphuric acids, as
should be the case with a true chloranhydride. In the reactions of
this chloranhydride we find the easiest means of introducing the
sulphonic group HSO3 into other compounds, because it is here combined
with chlorine. The second chloranhydride of sulphuric acid, or sulphuryl
chloride, SO2Cl2, was obtained by Regnault by the direct action of the
sun's ray on a mixture of equal volumes of chlorine and sulphurous
oxide. The gases gradually condense into a liquid, combining together
as carbonic oxide does with chlorine. It is also obtained when a mixture
of the two gases in acetic acid is allowed to stand for some time.
The first chloranhydride, SO3HCl, decomposes when heated at 200° in
a closed tube into sulphuric acid and sulphuryl chloride. It boils at
70°, its specific gravity is 1·7, it gives hydrochloric and sulphuric acids
with water, fumes in the air, and, judging by its vapour density, does
not decompose when distilled.[78]
[269]
In the group of the halogens we saw four closely analogous elements—fluorine,
chlorine, bromine, and iodine—and we meet with the same
number of closely allied analogues in the oxygen group; for besides[270]
[271]
sulphur this group also includes selenium and tellurium: O, S, Se, Te.
These two groups are very closely allied, both in respect to the magnitudes
of their atomic weights and also in the faculty of the elements
of both groups for combining with metals. The distinct analogy and
definite degree of variance known to us for the halogens, also repeat
themselves in the same degree for the elements of the oxygen group.
Amongst the halogens fluorine has many peculiarities compared to
Cl, Br and I which are more closely analogous, whilst oxygen differs in
many respects from S, Se, Te, which possess greater similarities. The
analogy in a quantitative respect is perfect in both cases. Thus the
halogens combine with H, and the elements of the oxygen group with
H2, forming H2O, H2S, H2Se, H2Te. The hydrogen compounds of
selenium and tellurium are acids like hydrogen sulphide. Selenium,
by simple heating in a stream of hydrogen, partially combines with it
directly, but seleniuretted hydrogen is more readily decomposable by
heat than sulphuretted hydrogen, and this property is still more
developed in telluretted hydrogen. Hydrogen selenide and telluride
are gases like sulphuretted hydrogen, and, like it, are soluble in water,
form saline compounds with alkalis, precipitate metallic salts, are
obtained by the action of acids on their compounds with metals, &c.
Selenium and tellurium, like sulphur, give two normal grades of combination
with oxygen, both of an acid character, of which only the
forms corresponding to sulphurous anhydride—namely, selenious anhydride,
SeO2, and tellurous anhydride, TeO2[79]—are formed directly.[272]
These are both solids, obtained by the combustion of the elements
themselves and by the action of oxidising agents on them. They form
feebly energetic acids, having distinct bibasic properties; however, a
characteristic difference from SO2 is observable both in the physical
properties of these compounds and in their stability and capacity for
further oxidation, just as in the series of the halogens already known to
us, only in an inverse order; in the latter we saw that iodine combines
more easily than bromine or chlorine with oxygen, forming more stable
oxygen compounds, whereas here, on the contrary, sulphurous anhydride,[273]
as we know, is difficultly decomposed, parts with its sulphur with
difficulty, and is easily oxidised and especially in its salts, while
selenious and tellurous anhydrides are oxidised with difficulty and
easily reduced, even by means of sulphurous acid.
Selenium was obtained in 1817 by Berzelius from the sublimate
which collects in the first chamber in the preparation of sulphuric
acid from Fahlun pyrites. Certain other pyrites also contain small
quantities of selenium. Some native selenides, especially those of lead,
mercury, and copper, have been found in the Hartz Mountains, but
only in small quantities. Pyrites and blendes, in which the sulphur
is partially replaced by selenium, still remain the chief source for its
extraction. When these pyrites are roasted they evolve selenious
anhydride, which condenses in the cooler portions of the apparatus in
which the pyrites are roasted, and is partially or wholly reduced by
the sulphurous anhydride simultaneously formed. The presence of
selenium in ores and sublimates is most simply tested by heating them
before the blowpipe, when they evolve the characteristic odour of garlic.
Selenium exhibits two modifications, like sulphur: one amorphous
and insoluble in carbon bisulphide, the other crystalline and slightly
soluble in carbon bisulphide (in 1,000 parts at 45° and 6,000 at 0°), and
separating from its solutions in monoclinic prisms. If the red precipitate
obtained by the action of sulphurous anhydride on selenious
anhydride be dried, it gives a brown powder, having a specific gravity
of 4·26, which when heated changes colour and fuses to a metallic
mass, which gains lustre as it cools. The selenium acquires different
properties according to the rate at which it is cooled from a fused
state; if rapidly cooled, it remains amorphous and has the same specific
gravity (4·28) as the powder, but if slowly cooled it becomes crystalline
and opaque, soluble in carbon bisulphide, and has a specific gravity
of 4·80. In this form it fuses at 214° and remains unchanged, whilst
the amorphous form, especially above 80°, gradually passes into the
crystalline variety. The transition is accompanied by the evolution
of heat, as in the case of sulphur; thus the analogy between sulphur
and selenium is clearly shown here. In the fused amorphous form
selenium presents a brown mass, slightly translucent, with a vitreous
fracture, whilst in the crystalline form it has the appearance of a grey
metal, with a feeble lustre and a crystalline fracture.[79 bis] Selenium[274]
boils at 700°, forming a vapour whose density is only constant at a temperature
of about 1,400°, when it is equal to 79·4 (referred to hydrogen)—that
is, the molecular formula is then Se2, like sulphur at an equally
high temperature.
Tellurium is met with still more rarely than selenium (it is known
in Saxony) in combination with gold, silver, lead, and antimony in
the so-called foliated tellurium ore. Bismuth telluride and silver
telluride have been found in Hungary and in the Altai. Tellurium is
extracted from bismuth telluride by mixing the finely-powdered ore
with potassium and charcoal in as intimate a mixture as possible,
and then heating in a covered crucible. Potassium telluride, K2Te,
is then formed, because the charcoal reduces potassium tellurite.
As potassium telluride is soluble in water, forming a red-brown
solution which is decomposed by the oxygen of the atmosphere
(K2Te + O + H2O = 2KHO + Te), the mass formed in the crucible is
treated with boiling water and filtered as rapidly as possible, and the
resultant solution exposed to the air, by which means the tellurium
is precipitated.[80] In a free state tellurium has a perfectly metallic
appearance; it is of a silver-white colour, crystallises very easily in
long brilliant needles; is very brittle, so that it can be easily reduced
to powder; but it is a bad conductor of heat and electricity, and
in this respect, as in many others, it forms a transition from the metals
to the non-metals. Its specific gravity is 6·18, it melts at an incipient
red heat, and takes fire when heated in air, like selenium and sulphur,
burning with a blue flame, evolving white fumes of tellurous anhydride,
TeO2, and emitting an acrid smell if no selenium be present;
but if it be, the odour of the latter preponderates. Alkalis dissolve
tellurium when boiled with it, potassium telluride, K2Te, and potassium
tellurite, K2TeO3, being formed. The solution is of a red colour, owing[275]
to the presence of the telluride, K2Te; but the colour disappears when
the solution is cooled or diluted, the tellurium being all precipitated:
2K2Te + K2TeO3 + 3H2O = 6KHO + 3Te.[81]
[276]
CHAPTER XXI
CHROMIUM, MOLYBDENUM, TUNGSTEN, URANIUM, AND MANGANESE
Sulphur, selenium, and tellurium belong to the uneven series of the
sixth group. In the even series of this group there are known chromium,
molybdenum, tungsten, and uranium; these give acid oxides
of the type RO3, like SO3. Their acid properties are less sharply
defined than those of sulphur, selenium, and tellurium, as is the case
with all elements of the even series as compared with those of the
uneven series in the same group. But still the oxides CrO3, MoO3,
WO3, and even UO3, have clearly defined acid properties, and form
salts of the composition MO,nRO3 with bases MO. In the case of the
heavy elements, and especially of uranium, the type of oxide, UO3,
is less acid and more basic, because in the even series of oxides the
element with the highest atomic weight always acquires a more and
more pronounced basic character. Hence UO3 shows the properties of
a base, and gives salts UO2X2. The basic properties of chromium,
molybdenum, tungsten, and uranium are most clearly expressed in the
lower oxides, which they all form. Thus chromic oxide, Cr2O3, is as
distinct a base as alumina, Al2O3.
Of all these elements chromium is the most widely distributed
and the most frequently used. It gives chromic anhydride, CrO3, and
chromic oxide, Cr2O3—two compounds whose relative amounts of
oxygen stand in the ratio 2 : 1. Chromium is, although somewhat
rarely, met with in nature as a compound of one or the other type.
The red chromium ore of the Urals, lead chromate or crocoisite
PbCrO4, was the source in which chromium was discovered by
Vauquelin, who gave it this name (from the Greek word signifying
colour) owing to the brilliant colours of its compounds; the chromates
(salts of chromic anhydride) are red and yellow, and the chromic salts
(from Cr2O3) green and violet. The red lead chromate is, however, a
rare chromium ore found only in the Urals and in a few other localities.
Chromic oxide, Cr2O3, is more frequently met with. In small quantities
it forms the colouring matter of many minerals and rocks—for example,[277]
of some serpentines. The commonest ore, and the chief source of the
chromium compounds, is the chrome iron ore or chromite, which occurs
in the Urals[1] and Asia Minor, California, Australia, and other
localities. This is magnetic iron ore, FeO,Fe2O3, in which the ferric
oxide is replaced by chromic oxide, its composition being FeO,Cr2O3.
Chrome iron ore crystallises in octahedra of sp. gr. 4·4; it has a feeble
metallic lustre, is of a greyish-black colour, and gives a brown powder.
It is very feebly acted on by acids, but when fused with potassium
acid sulphate it gives a soluble mass, which contains a chromic salt,
besides potassium sulphate and ferrous sulphate. In practice the
treatment of chrome iron ore is mainly carried on for the preparation
of chromates, and not of chromic salts, and therefore we will trace the
history of the element by beginning with chromic acid, and especially
with the working up of the chrome iron ore into potassium dichromate,
K2Cr2O7, as the most common salt of this acid. It must be remarked
that chromic anhydride, CrO3, is only obtained in an anhydrous state,
and is distinguished for its capacity for easily giving anhydro-salts
with the alkalis, containing one, two, and even three equivalents of the
anhydride to one equivalent of base. Thus among the potassium salts
there is known the normal or yellow chromate, K2CrO4, which corresponds
to, and is perfectly isomorphous with, potassium sulphate, easily
forms isomorphous mixtures with it, and is not therefore suitable for a
process in which it is necessary to separate the salt from a mixture
containing sulphates. As in the presence of a certain excess of acid,
the dichromate, K2Cr2O7 = 2K2CrO4 + 2HX - 2KX - H2O, is easily
formed from K2CrO4, the object of the manufacturer is to produce
such a dichromate, the more so as it contains a larger proportion of the
elements of chromic acid than the normal salt. Finely-ground chrome
iron ore, when heated with an alkali, absorbs oxygen almost as easily
(Chapter III., Note 7) as a mixture of the oxides of manganese with
an alkali. This absorption is due to the presence of chromic oxide,
which is oxidised into the anhydride, and then combines with the
alkali Cr2O3 + O3 = 2CrO3. As the oxidation and formation of the
chromate proceeds, the mass turns yellow. The iron is also oxidised,
but does not give ferric acid, because the capacity of the chromium for
oxidation is incomparably greater than that of the iron.
A mixture of lime (sometimes with potash) and chrome iron ore
is heated in a reverberatory furnace, with free access of air and at a[278]
red heat for several hours, until the mass becomes yellow; it then
contains normal calcium chromate, CaCr_O4, which is insoluble in
water in the presence of an excess of lime.[1 bis] The resultant mass is
ground up, and treated with water and sulphuric acid. The excess of
lime forms gypsum, and the soluble calcium dichromate, CaCr2O7,
together with a certain amount of iron, pass into solution. The
solution is poured off, and chalk added to it; this precipitates the
ferric oxide (the ferrous oxide is converted into ferric oxide in the
furnace) and forms a fresh quantity of gypsum, while the chromic acid
remains in solution—that is, it does not form the sparingly-soluble
normal salt (1 part soluble in 240 parts of water). The solution then
contains a fairly pure calcium dichromate, which by double decomposition
gives other chromates; for example, with a solution of potassium
sulphate it gives a precipitate of calcium sulphate and a solution of
potassium dichromate, which crystallises when evaporated.[2]
Potassium dichromate, K2Cr2O7, easily crystallises from acid solutions
in red, well-formed prismatic crystals, which fuse at a red heat
and evolve oxygen at a very high temperature, leaving chromic oxide
and the normal salt, which undergoes no further change: 2K2Cr2O7
= 2K2CrO4 + Cr2O3 + O3. At the ordinary temperature 100 parts
of water dissolve 10 parts of this salt, and the solubility increases as
the temperature rises. It is most important to note that the
dichromate does not contain water, it is K2CrO4 + CrO3; the acid
salt corresponding to potassium acid sulphate, KHSO4, does not exist.
It does not even evolve heat when dissolving in water, but on the contrary
produces cold, i.e. it does not form a very stable compound with
water. The solution and the salt itself are poisonous, and act as
powerful oxidising agents, which is the character of chromic acid in
general. When heated with sulphur or organic substances, with
sulphurous anhydride, hydrogen sulphide, &c., this salt is deoxidised,
yielding chromic compounds.[2 bis] Potassium dichromate[3] is used in the
arts and in chemistry as a source for the preparation of all other[279]
chromium compounds. It is converted into yellow pigments by means
of double decomposition with salts of lead, barium, and zinc. When
solutions of the salts of these metals are mixed with potassium
dichromate (in dyeing generally mixed with soda, in order to obtain
normal salts), they are precipitated as insoluble normal salts; for
example, 2BaCl2 + K2Cr2O7 + H2O = 2BaCrO4 + 2KCl + 2HCl. It
follows from this that these salts are insoluble in dilute acids, but
the precipitation is not complete (as it would be with the normal salt).
The barium and zinc salts are of a lemon yellow colour; the lead salt
has a still more intense colour passing into orange. Yellow cotton
prints are dyed with this pigment. The silver salt, Ag2CrO4, is of a
bright red colour.
When potassium dichromate is mixed with potassium hydroxide[280]
or carbonate (carbonic anhydride being disengaged in the latter case) it
forms the normal salt, K2CrO4, known as yellow chromate of potassium.
Its specific gravity is 2·7, being almost the same as that of the dichromate.
It absorbs heat in dissolving; one part of the salt dissolves in
1·75 part of water at the ordinary temperature, forming a yellow
solution. When mixed even with such feeble acids as acetic, and more
especially with the ordinary acids, it gives the dichromate, and Graham
obtained a trichromate, K2Cr3O10 = K2CrO4,2CrO3, by mixing a
solution of the latter salt with an excess of nitric acid.
Chromic anhydride is obtained by preparing a saturated solution of
potassium dichromate at the ordinary temperature, and pouring it in a
thin stream into an equal volume of pure sulphuric acid.[4] On mixing,
the temperature naturally rises; when slowly cooled, the solution
deposits chromic anhydride in needle-shaped crystals of a red colour
sometimes several centimetres long. The crystals are freed from the
mother liquor by placing them on a porous tile.[4 bis] It is very important
at this point to call attention to the fact that a hydrate of chromic
anhydride is never obtained in the decomposition of chromic compounds,[281]
but always the anhydride, CrO3. The corresponding hydrate, CrO4H2,
or any other hydrate, is not even known. Nevertheless, it must be
admitted that chromic acid is bibasic, because it forms salts isomorphous
or perfectly analogous with the salts formed by sulphuric acid, which is
the best example of a bibasic acid. A clear proof of the bibasicity of
CrO3 is seen in the fact that the anhydride and salts give (when heated
with sodium chloride and sulphuric acid) a volatile chloranhydride,
CrO2Cl2, containing two atoms of chlorine as a bibasic acid should.[5][282]
Chromic anhydride is a red crystalline substance, which is converted
into a black mass by heat; it fuses at 190°, and disengages oxygen
above 250°, leaving a residue of chromium dioxide, CrO2,[6] and, on still
further heating, chromic oxide, Cr2O3. Chromic anhydride is exceedingly
soluble in water, and even attracts moisture from the air, but, as
was mentioned above, it does not form any definite compound with
water. The specific gravity of its crystals is 2·7, and when fused it has
a specific gravity 2·6. The solution presents perfectly defined acid
properties. It liberates carbonic anhydride from carbonates; gives
insoluble precipitates of the chromates with salts of barium, lead, silver,
and mercury.
The action of hydrogen peroxide on a solution of chromic acid or of
potassium dichromate gives a blue solution, which very quickly becomes
colourless with the disengagement of oxygen. Barreswill showed that
this is due to the formation of a perchromic anhydride, Cr2O7, corresponding
with sulphur peroxide. This peroxide is remarkable from the
fact that it very easily dissolves in ether and is much more stable in
this solution, so that, by shaking up hydrogen peroxide mixed with a
small quantity of chromic acid, with ether, it is possible to transfer all
the blue substance formed to the ether.[6 bis]
With oxygen acids, chromic acid evolves oxygen; for example, with[283]
sulphuric acid the following reaction takes place: 2CrO3 + 3H2SO4
= Cr2(SO4)3 + O3 + 3H2O. It will be readily understood from this that
a mixture of chromic acid or of its salts with sulphuric acid forms an
excellent oxidising agent, which is frequently employed in chemical
laboratories and even for technical purposes as a means of oxidation.
Thus hydrogen sulphide and sulphurous anhydride are converted into
sulphuric acid by this means. Chromic acid is able to act as a powerful
oxidising agent because it passes into chromic oxide, and in so doing
disengages half of the oxygen contained in it: 2CrO3 = Cr2O3 + O3.
Thus chromic anhydride itself is a powerful oxidising agent, and is
therefore employed instead of nitric acid in galvanic batteries (as a
depolariser), the hydrogen evolved at the carbon being then oxidised,
and the chromic acid converted into a non-volatile product of deoxidation,
instead of yielding, as nitric acid does, volatile lower oxides of
offensive odour. Organic substances are more or less perfectly oxidised
by means of chromic anhydride, although this generally requires the aid
of heat, and does not proceed in the presence of alkalis, but generally
in the presence of acids. In acting on a solution of potassium iodide,
chromic acid, like many oxidising agents, liberates iodine; the reaction
proceeds in proportion to the amount of CrO3 present, and may serve
for determining the amount of CrO3, since the amount of iodine liberated
can be accurately determined by the iodometric method (Chapter XX.,
Note 42). If chromic anhydride be ignited in a stream of ammonia, it
gives chromic oxide, water, and nitrogen. In all cases when chromic
acid acts as an oxidising agent in the presence of acids and under the
action of heat, the product of its deoxidation is a chromic salt, CrX3,
which is characterised by the green colour of its solution, so that the
red or yellow solution of a salt of chromic acid is then transformed into
a green solution of a chromic salt, derived from chromic oxide, Cr2O3,
which is closely analogous to Al2O3, Fe2O3, and other bases of the composition
R2O3. This analogy is seen in the insolubility of the anhydrous
oxide, in the gelatinous form of the colloidal hydrate, in the formation
of alums,[7] of a volatile chloride of chromium, &c.[7 bis]
[284]
[285]
[286]
Chromic oxide, Cr2O3, rarely found, and in small quantities, in chrome
ochre, is formed by the oxidation of chromium and its lower oxides, by
the reduction of chromates (for example, of ammonium or mercuric
chromate) and by the decomposition (splitting up) of the saline compounds
of the oxide itself, CrX3 or Cr2X6, like alumina, which it
resembles in forming a feeble base easily giving double and basic salts,
which are either green or violet.
[287]
[288]
[289]
The reduction of chromic oxide—for instance, in a solution by zinc
and sulphuric acid—leads to the formation of chromous oxide, CrO, and
its salts, CrX2, of a blue colour (see Notes 7 and 7bis). The further
reduction[8] of oxide of chromium and its corresponding compounds
gives metallic chromium. Deville obtained it (probably containing
carbon) by reducing chromic oxide with carbon, at a temperature near
the melting point of platinum, about 1750°, but the metal itself does
not fuse at this temperature. Chromium has a steel-grey colour and is
very hard (sp. gr. 5·9), takes a good polish, and dissolves in hydrochloric
acid, but cold dilute sulphuric and nitric acids have no action
upon it. Bunsen obtained metallic chromium by decomposing a solution
of chromic chloride, Cr2Cl6, by a galvanic current, as scales of a grey
colour (sp. gr. 7·3). Wöhler obtained crystalline chromium by igniting
a mixture of the anhydrous chromic chloride Cr2Cl6 (see Note 7 bis)
with finely-divided zinc, and sodium and potassium chlorides, at the
boiling-point of zinc. When the resultant mass has cooled the zinc may[290]
be dissolved in dilute nitric acid, and grey crystalline chromium (sp. gr.
6·81) is left behind. Frémy also prepared crystalline chromium by the
action of the vapour of sodium on anhydrous chromic chloride in a
stream of hydrogen, using the apparatus shown in the accompanying
drawing, and placing the sodium and the chromic chloride in separate
porcelain boats. The tube containing these boats is only heated when
it is quite full of dry hydrogen. The crystals of metallic chromium
obtained in the tube are grey cubes having a considerable hardness and
withstanding the action of powerful acids, and even of aqua regia.
The chromium obtained by Wöhler by the action of a galvanic current
is, on the contrary, acted on under these conditions. The reason of
this difference must be looked for in the presence of impurities, and in
the crystalline structure. But in any case, among the properties of
metallic chromium, the following may be considered established: it is
white in colour, with a specific gravity of about 6·7, is extremely hard
in a crystalline form, is not oxidised by air at the ordinary temperature,
and with carbon it forms alloys like cast iron and steel.
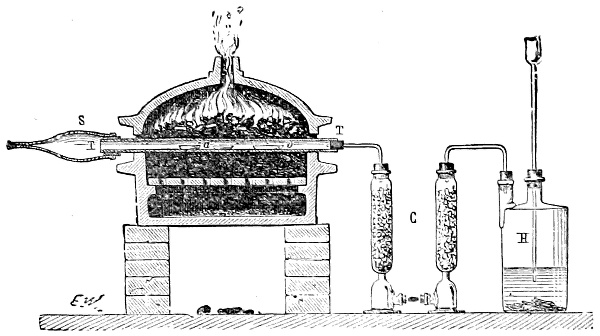
Fig. 92.—Apparatus for the preparation of metallic chromium by igniting chromic chloride
and sodium in a stream of hydrogen.
The two analogues of chromium, molybdenum and tungsten (or wolfram),
are of still rarer occurrence in nature, and form acid oxides, RO3,
which are still less energetic than CrO3. Tungsten occurs in the somewhat
rare minerals, scheelite, CaWO4, and wolfram; the latter being an
isomorphous mixture of the normal tungstates of iron and manganese,
(MnFe)WO4. Molybdenum is most frequently met with as molybdenite,
MoS2, which presents a certain resemblance to graphite in its physical
properties and softness. It also occurs, but much more rarely, as a
yellow lead ore, PbMoO4. In both these forms molybdenum occurs in
the primary rocks, in granites, gneiss, &c., and in iron and copper ores[291]
in Saxony, Sweden, and Finland. Tungsten ores are sometimes met
with in considerable masses in the primary rocks of Bohemia and
Saxony, and also in England, America, and the Urals. The preliminary
treatment of the ore is very simple; for example, the sulphide,
MoS2, is roasted, and thus converted into sulphurous anhydride and
molybdic anhydride, MoO3, which is then dissolved in alkalis, generally
in ammonia. The ammonium molybdate is then treated with acids,
when the sparingly soluble molybdic acid is precipitated. Wolfram is
treated in a different manner. Most frequently the finely-ground ore is
repeatedly boiled with hydrochloric and nitric acids, and the resultant
solutions (of salts of manganese and iron) poured off, until the dark
brown mass of ore disappears, whilst the tungstic acid remains, mixed
with silica, as an insoluble residue; it is treated also with ammonia,
and is thus converted into soluble ammonium tungstate, which passes
into solution and yields tungstic acid when treated with acids. This
hydrate is then ignited, and leaves tungstic anhydride. The general
character of molybdic and tungstic anhydrides is analogous to that of
chromic anhydride; they are anhydrides of a feebly acid character,
which easily give polyacid salts and colloid solutions.[8 bis]
[292]
[293]
[294]
[295]
[296]
Hydrogen (which does not directly form compounds with Cr, Mo,
and W) reduces molybdic and tungstic anhydride at a red heat; and
this forms the means of obtaining metallic molybdenum and tungsten.
Both metals are infusible, and both under the action of heat form
compounds with carbon and iron (the addition of tungsten to steel
renders the latter ductile and hard).[9] Molybdenum forms a grey powder,
which scarcely aggregates under a most powerful heat, and has a specific
gravity of 8·5. It is not acted on by the air at the ordinary temperature,
but when ignited it is first converted into a brown, and then into a
blue oxide, and lastly into molybdic anhydride. Acids do not act on it—that
is, it does not liberate hydrogen from them, not even from
hydrochloric acid—but strong sulphuric acid disengages sulphurous
anhydride, forming a brown mass, containing a lower oxide of molybdenum.
Alkalis in solution do not act on molybdenum, but when fused[297]
with it hydrogen is given off, which shows, as does its whole character,
the acid properties of the metal. The properties of tungsten are almost
identical; it is infusible, has an iron-grey colour, is exceedingly hard, so
that it even scratches glass. Its specific gravity is 19·1 (according to
Roscoe), so that, like uranium, platinum, &c., it is one of the heaviest
metals.[9 bis] Just as sulphur and chromium have their corresponding
persulphuric and perchromic acids, H2S2O8 and H2CrO8, having the
properties of peroxides, and corresponding to peroxide of hydrogen, so
also molybdenum and tungsten are known to give permolybdic and pertungstic
acids, H2Mo2O8 and H2W2O8, which have the properties of true
peroxides, i.e. easily disengage iodine from KI and chlorine from HCl,
easily part with their oxygen, and are formed by the action of peroxide
of hydrogen, into which they are readily reconverted (hence they may
be regarded as compounds of H2O2 with 2MoO3 and 2WO3), &c. Their
formation (Boerwald 1884, Kemmerer 1891) is at once seen in the
coloration (not destroyed by boiling), which is obtained on mixing a
solution of the salts with peroxide of hydrogen, and on treating, for instance,
molybdic acid with a solution of peroxide of hydrogen (Péchard
1892). The acid then forms an orange-coloured solution, which after
evaporation in vacuo leaves Mo2H2O84H2O as a crystalline powder,
and loses 4H2O at 100°, beyond which it decomposes with the evolution
of oxygen.[9 tri]
Uranium, U = 240, has the highest atomic weight of all the
analogues of chromium, and indeed of all the elements yet known. Its[298]
highest salt-forming oxide, UO3, shows very feeble acid properties.
Although it gives sparingly-soluble yellow compounds with alkalis,
which fully correspond with the dichromates—for example, Na2U2O7
= Na2O,2UO3,[10]—yet it more frequently and easily reacts with acids, HX,[299]
forming fluorescent yellowish-green salts of the composition UO2X2,
and in this respect uranic trioxide, UO3, differs from chromic anhydride,
CrO3, although the latter is able to give the oxychloride, CrO2Cl2. In
molybdenum and tungsten, however, we see a clear transition from
chromium to uranium. Thus, for example, chromyl chloride, CrO2Cl2,
is a brown liquid which volatilises without change, and is completely
decomposed by water; molybdenum oxychloride, MoO2Cl2, is a crystalline
substance of a yellow colour, which is volatile and soluble in
water (Blomstrand), like many salts. Tungsten oxychloride, WO2Cl2,
stands still nearer to uranyl chloride in its properties; it forms yellow
scales on which water and alkalis act, as they do on many salts (zinc
chloride, ferric chloride, aluminium chloride, stannic chloride, &c.), and
perfectly corresponds with the difficultly volatile salt, UO2Cl2 (obtained
by Peligot by the action of chlorine on ignited uranium dioxide, UO2),
which is also yellow and gives a yellow solution with water, like all the[300]
salts UO2X2. The property of uranic oxide, UO3, of forming salts
UO2X2 is shown in the fact that the hydrated oxide of uranium,
UO2(HO)2, which is obtained from the nitrate, carbonate, and other
salts by the loss of the elements of the acid, is easily soluble in acids,
as well as in the fact that the lower grades of oxidation of uranium are
able, when treated with nitric acid, to form an easily crystallisable
uranyl nitrate, UO2(NO3)2,6H2O; this is the most commonly occurring
uranium salt.[11]
Uranium, which gives an oxide, UO3, and the corresponding salt
UO2X2 and dioxide UO2, to which the salts UX4 correspond, is rarely
met with in nature. Uranite or the double orthophosphate of uranic[301]
oxide, R(UO2)H2P2O8,7H2O, where R = Cu or Ca, uranium-vitriol
U(SO4)2,H2O, samarakite, and æschynite, are very rarely found, and
then only in small quantities. Of more frequent and abundant
occurrence is the non-crystalline, earthy brown uranium ore known as
pitchblende (sp. gr. 7·2), which is mainly composed of the intermediate
oxide, U3O8 = UO2,2UO3. This ore is found at Joachimsthal in Bohemia
and in Cornwall. It usually contains a number of different impurities,
chiefly sulphides and arsenides of lead and iron, as well as lime
and silica compounds. In order to expel the arsenic and sulphur it is
roasted, ground, washed with dilute hydrochloric acid, which does not
dissolve the uranoso-uranic oxide, U3O8, and the residue is dissolved
in nitric acid, which transforms the uranium oxide into the nitrate,
UO2(NO3)2.
It must be observed that the oxide of uranium, first distinguished
by Klaproth (1789), was for a long time regarded as able to give
metallic uranium under the action of charcoal and other reducing agents
(with the aid of heat). But the substance thus obtained was only the
uranium dioxide, UO2. The compound nature of this dioxide,[12] or the
presence of oxygen in it, was demonstrated by Peligot (1841), by igniting
it with charcoal in a stream of chlorine. He thus obtained a volatile
uranium tetrachloride, UCl4,[13] which, when heated with sodium, gave[302]
metallic uranium as a grey metal, having a specific gravity of 18·7, and
liberating hydrogen from acids, with the formation of green uranous
salts, UX4, which act as powerful reducing agents.[14]
[303]
As the salts of uranic oxide are reduced in the absence of organic
matter by the action of light, and as they impart a characteristic
coloration to glass,[15] they find a certain application in photography and
glass work.
If we compare together the highly acid elements, sulphur, selenium,
and tellurium, of the uneven series, with chromium, molybdenum,
tungsten, and uranium of the even series, we find that the resemblance
of the properties of the higher form RO3 does not extend to the lower
forms, and even entirely disappears in the elements, for there is
not the smallest resemblance between sulphur and chromium and their
analogues in a free state. In other words, this means that the small
periods, like Na, Mg, Al, Si, P, S, Cl, containing seven elements, do
not contain any near analogues of chromium, molybdenum, &c., and
therefore their true position among the other elements must be looked
for only in those large periods which contain two small periods, and
whose type is seen in the period containing: K, Ca, Sc, Ti, V, Cr, Mn,
Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br. These large periods contain
Ca and Zn, giving RO, Sc, and Ga of the third group, Ti and Ge
giving RO2, V and As forming R2O5, Cr and Se of the sixth group,
Mn and Br of the seventh group, and the remaining elements, Fe,
Co, Ni, form connective members of the intermediate eighth group, to
the description of the representatives of which we shall turn in the
following chapters. We will now proceed to describe manganese,
Mn = 55, as an element of the seventh group of the even series, directly
following after Cr = 52, which corresponds with Br = 80 to the same
degree that Cr does with Se = 79. For chromium, selenium, and
bromine very close analogues are known, but for manganese as yet
none have been obtained—that is, it is the only representative of the
even series in the seventh group. In placing manganese with the[304]
halogens in one group, the periodic system of the elements only requires
that it should bear an analogy to the halogens in the higher type of
oxidation—i.e. in the salts and acids—whilst it requires that as great
a difference should be expected in the lower types and elements as there
exists between chromium or molybdenum and sulphur or selenium.
And this is actually the case. The elements of the seventh group form
a higher salt-forming oxide, R2O7, and its corresponding hydrate,
HRO4, and salts—for example, KClO4. Manganese in the form of
potassium permanganate, KMnO4, actually presents a great analogy in
many respects to potassium perchlorate, KClO4. The analogy of the
crystalline form of both salts was shown by Mitscherlich. The salts of
permanganic acid are also nearly all soluble in water, like those of
perchloric acid, and if the silver salt of the latter, AgClO4, be sparingly
soluble in water, so also is silver permanganate, AgMnO4. The specific
volume of potassium perchlorate is equal to 55, because its specific
gravity = 2·54; the specific volume of potassium permanganate is equal
to 58, because its specific gravity = 2·71. So that the volumes of
equivalent quantities are in this instance approximately the same
whilst the atomic volumes of chlorine (35·5/1·3 = 27) and manganese
(55/7·5) are in the ratio 4 : 1. In a free state the higher acids HClO4
and HMnO4 are both soluble in water and volatile, both are powerful
oxidisers—in a word, their analogy is still closer than that of chromic
and sulphuric acids, and those points of distinction which they present
also appear among the nearest analogues—for example, in sulphuric and
telluric acids, in hydrochloric and hydriodic acids, &c. Besides Mn2O7
manganese gives a lower grade of oxidation, MnO3, analogous to
sulphuric and chromic trioxides, and with it corresponds potassium
manganate, K2MnO4, isomorphous with potassium sulphate.[16] In the
still lower grades of oxidation, Mn2O3 and MnO, there is hardly any
similarity to chlorine, whilst every point of resemblance disappears
when we come to the elements themselves—i.e. to manganese and
chlorine—for manganese is a metal, like iron, which combines directly
with chlorine to form a saline compound, MnCl2, analogous to magnesium
chloride.[17]
Manganese belongs to the number of metals widely distributed in[305]
nature, especially in those localities where iron occurs, whose ores
frequently contain compounds of manganous oxide, MnO, which presents
a resemblance to ferrous oxide, FeO, and to magnesia. In many minerals
magnesia and the oxides allied to it are replaced by manganous oxide;
calcspars and magnesites—i.e. R″CO3 in general—are frequently met
with containing manganous carbonate, which also occurs in a separate
state, although but rarely. The soil also and the ash of plants generally
contain a small quantity of manganese. In the analysis of minerals
it is generally found that manganese occurs together with magnesia,
because, like it, manganous oxide remains in solution in the presence of
ammoniacal salts, not being precipitated by reagents. The property of
this manganous oxide, MnO, of passing into the higher grades of oxidation
under the influence of heat, alkalis, and air, gives an easy means
not only of discovering the presence of manganese in admixture with
magnesia, but also of separating these two analogous bases. Magnesia is
not able to give higher grades of oxidation, whilst manganese gives them
with great facility. Thus, for instance, an alkaline solution of sodium
hypochlorite produces a precipitate of manganese dioxide in a solution of
a manganous salt: MnCl2 + NaClO + 2NaHO = MnO2 + H2O + 3NaCl;
whilst magnesia is not changed under these circumstances, and remains
in the form of MgCl2. If the magnesia be precipitated owing to the
presence of alkali, it may be dissolved in acetic acid, in which manganese
dioxide is insoluble. The presence of small quantities of manganese
may also be recognised by the green coloration which alkalis acquire
when heated with manganese compounds in the air. This green coloration
depends on the property of manganese of giving a green alkaline
manganate: MnCl2 + 4KHO + O2 = K2MnO4 + 2KCl + 2H2O. Thus
the faculty of oxidising in the presence of alkalis forms an essential
character of manganese. The higher grades of oxidation containing
Mn2O7 and MnO3 are quite unknown in nature, and even MnO2 is not
so widely spread in nature as the ores composed of manganous compounds
which are met with nearly everywhere. The most important
ore of manganese is its dioxide, or so-called peroxide, MnO2, which is
known in mineralogy as pyrolusite. Manganese also occurs as an
oxide corresponding with magnetic iron ore, MnO,Mn2O3 = Mn3O4,
forming the mineral known as hausmannite. The oxide Mn2O3 also
occurs in nature as the anhydrous mineral braunite, and in a hydrated
form, Mn2O3,H2O, called manganite. Both of these often occur as an
admixture in pyrolusite. Besides which, manganese is met with in
nature as a rose-coloured mineral, rhodonite, or silicate, MnSiO3. Very
fine and rich deposits of manganese ores have been found in the
Caucasus, the Urals, and along the Dnieper. Those at the Sharapansky[306]
district of the Government of Kutais and at Nicopol on the Dnieper
are particularly rich. A large quantity of the ore (as much as 100,000
tons yearly) is exported from these localities.
Thus manganese gives oxides of the following forms: MnO,
manganous oxide, and manganous salts, MnX2, corresponding with the
base, which resembles magnesia and ferrous oxide in many respects;
Mn2O3, a very feeble base, giving salts, MnX3, analogous to the
aluminium and ferric salts, easily reduced to MnX2; MnO2, dioxide,
generally called peroxide, an almost indifferent oxide, or feebly acid;[18]
MnO3, manganic anhydride, which forms salts resembling potassium
sulphate;[18 bis] Mn2O7, permanganic anhydride, giving salts analogous
to the perchlorates.
All the oxides of manganese when heated with acids give salts, MnX2,
corresponding with the lower grade of oxidation, manganous oxide,
MnO. Manganic oxide, Mn2O3, is a feebly energetic base; it is true
that it dissolves in hydrochloric acid and gives a dark solution containing
the salt MnCl3, but the latter when heated evolves chlorine
and gives a salt corresponding with manganous oxide MnCl2—i.e. at
first: Mn2O3 + 6HCl = 3H2O + Mn2Cl6, and then the Mn2Cl6 decomposes
into 2MnCl2 + Cl2. None of the remaining higher grades of
oxidation have a basic character, but act as oxidising agents in the
presence of acids, disengaging oxygen and passing into salts of the lower
grade of oxidation of manganese, MnO. Owing to this circumstance,
the manganous salts are often obtained; they are, for instance, left in
the residue when the dioxide is used for the preparation of oxygen and
chlorine.[19]
[307]
As the salts of manganous oxide MnX2 closely resemble (and are
isomorphous with) the salts of magnesia MgX2 in many respects (with
the exception of the fact that MnX2 are rose coloured and are easily
oxidised in the presence of alkalis), we will not dwell upon them, but[308]
[309]
limit ourselves to illustrating the chemical character of manganese by
describing the metal and its corresponding acids. The fact alone that
the oxides of manganese are not reduced to the metal when ignited in
hydrogen (whilst the oxides of iron give metallic iron under these
circumstances), but only to manganous oxide, MnO, shows that
manganese has a considerable affinity for oxygen—that is, it is difficult
to reduce. This may be effected, however, by means of charcoal or
sodium at a very high temperature. A mixture of one of the oxides of
manganese with charcoal or organic matter gives fused metallic manganese
under the powerful heat developed by coke with an artificial
draught. The metal was obtained for the first time in this manner by
Gahn, after Pott, and more especially Scheele, had in the last century
shown the difference between the compounds of iron and manganese
(they were previously regarded as being the same). Manganese is prepared
by mixing one of its oxides in a finely-divided state with oil and
soot; the resultant mass is then first ignited in order to decompose
the organic matter, and afterwards strongly heated in a charcoal crucible.
The manganese thus obtained, however, contains, as a rule, a considerable
amount of silicon and other impurities. Its specific gravity varies
between 7·2 and 8·0. It has a light grey colour, a feebly metallic
lustre, and although it is very hard it can be scratched by a file. It
rapidly oxidises in air, being converted into a black oxide; water acts
on it with the evolution of hydrogen—this decomposition proceeds very
rapidly with boiling water, and if the metal contain carbon.[20]
[310]
It has been shown above that if manganese dioxide, or any
lower oxide of manganese, be heated with an alkali in the presence of
air, the mixture absorbs oxygen,[21] and forms an alkaline manganate of a
green colour: 2KHO + MnO2 + O = K2MnO4 + H2O. Steam is disengaged
during the ignition of the mixture, and if this does not take
place there is no absorption of oxygen. The oxidation proceeds much
more rapidly if, before igniting in air, potassium chlorate or nitre be
added to the mixture, and this is the method of preparing potassium
manganate, K2MnO4. The resultant mass dissolved in a small quantity
of water gives a dark green solution, which, when evaporated under the
receiver of an air-pump over sulphuric acid, deposits green crystals of
exactly the same form as potassium sulphate—namely, six-sided prisms
and pyramids. The composition of the product is not changed by being
re-dissolved, if perfectly pure water free from air and carbonic acid be
taken. But in the presence of even very feeble acids the solution of
this salt changes its colour and becomes red, and deposits manganese
dioxide. The same decomposition takes place when the salt is heated
with water, but when diluted with a large quantity of unboiled water
manganese dioxide does not separate, although the solution turns red.
This change of colour depends on the fact that potassium manganate,
K2MnO4, whose solution is green, is transformed into potassium permanganate,
KMnO4, whose solution is of a red colour. The reaction
proceeding under the influence of acids and a large quantity of water[311]
is expressed in the following manner: 3K2MnO4 + 2H2O = 2KMnO4
+ MnO2 + 4KHO. If there is a large proportion of acid and the decomposition
is aided by heat, the manganese dioxide and potassium
permanganate are also decomposed, with formation of manganous salt.
Exactly the same decomposition as takes place under the action of acids
is also accomplished by magnesium sulphate, which reacts in many cases
like an acid. When water holding atmospheric oxygen in solution acts
on a solution of potassium manganate, the oxygen combines directly
with the manganate and forms potassium permanganate, without
precipitating manganese dioxide, 2K2MnO4 + O + H2O = 2KMnO4
+ 2KHO. Thus a solution of potassium manganate undergoes a very
characteristic change in colour and passes from green to red; hence this
salt received the name of chameleon mineral.[22]
Potassium permanganate, KMnO4, crystallises in well-formed, long
red prisms with a bright green metallic lustre. In the arts the potash
is frequently replaced by soda, and by other alkaline bases, but no salt
of permanganic acid crystallises so well as the potassium salt, and
therefore this salt is exclusively used in chemical laboratories. One
part of the crystalline salt dissolves in 15 parts of water at the ordinary
temperature. The solution is of a very deep red colour, which is so
intense that it is still clearly observable after being highly diluted with
water. In a solid state it is decomposed by heat, with evolution of[312]
oxygen, a residue consisting of the lower oxides of manganese and
potassium oxide being left.[22 bis] A mixture of permanganate of potassium,
phosphorous and sulphur takes fire when struck or rubbed, a
mixture of the permanganate with carbon only takes fire when heated,
not when struck. The instability of the salt is also seen in the fact
that its solution is decomposed by peroxide of hydrogen, which at the
same time it decomposes. A number of substances reduce potassium
permanganate to manganese dioxide (in which case the red solution
becomes colourless).[23] Many organic substances (although far from
all, even when boiled in a solution of permanganate) act in this manner,
being oxidised at the expense of a portion of its oxygen. Thus, a
solution of sugar decomposes a cold solution of potassium permanganate.
In the presence of an excess of alkali, with a small quantity of sugar,
the reduction leads to the formation of potassium manganate, because
2KMnO4 + 2KHO = O + 2K2MnO4 + H2O. With a considerable amount
of sugar and a more prolonged action, the solution turns brown and
precipitates manganese dioxide or even oxide. In the oxidation of
many organic bodies by an alkaline solution of KMnO4 generally three-eighths
of the oxygen in the salt are utilised for oxidation: 2KMnO4
= K2O + 2MnO2 + O3. A portion of the alkali liberated is retained by
the manganese dioxide, and the other portion generally combines with
the substance oxidised, because the latter most frequently gives an acid
with an excess of alkali. A solution of potassium iodide acts in a
similar manner, being converted into potassium iodate at the expense of
the three atoms of oxygen disengaged by two molecules of potassium
permanganate.
In the presence of acids, potassium permanganate acts as an oxidising
agent with still greater energy than in the presence of alkalis. At any
rate, a greater proportion of oxygen is then available for oxidation,
namely, not ⅜, as in the presence of alkalis, but ⅝, because in the first
instance manganese dioxide is formed, and in the second case manganous
oxide, or rather the salt, MnX2, corresponding with it. Thus, for[313]
instance, in the presence of an excess of sulphuric acid, the decomposition
is accomplished in the following manner: 2KMnO4 + 3H2SO4
= K2SO4 + 2MnSO4 + 3H2O + 5O. This decomposition, however, does
not proceed directly on mixing a solution of the salt with sulphuric
acid, and crystals of the salt even dissolve in oil of vitriol without the
evolution of oxygen, and this solution only decomposes by degrees after
a certain time. This is due to the fact that sulphuric acid liberates
free permanganic acid from the permanganate,[24] which acid is stable
in solution. But if, in the presence of acids and a permanganate, there[314]
is a substance capable of absorbing oxygen—for instance, capable of
passing into a higher grade of oxidation—then the reduction of the
permanganic acid into manganous oxides sometimes proceeds directly
at the ordinary temperature. This reduction is very clearly seen,
because the solutions of potassium permanganate are red whilst the
manganous salts are almost colourless. Thus, for instance, nitrous acid
and its salts are converted into nitric acid and decolorise the acid solution
of the permanganate. Sulphurous anhydride and its salts immediately
decolorise potassium permanganate, forming sulphuric acid. Ferrous
salts, and in general salts of lower grades of oxidation capable of being
oxidised in solution, act in exactly the same manner. Sulphuretted
hydrogen is also oxidised to sulphuric acid; even mercury is oxidised
at the expense of permanganic acid, and decolorises its solution, being
converted into mercuric oxide. Moreover, the end point of these reactions
may easily be seen, and therefore, having first determined the amount
of active oxygen in one volume of a solution of potassium permanganate,
and knowing how many volumes are required to effect a given oxidation,
it is easy to determine the amount of an oxidisable substance in a
solution from the amount of permanganate expended (Marguerite's
method).
The oxidising action of KMnO4, like all other chemical reactions,
is not accomplished instantaneously, but only gradually. And, as the
course of the reaction is here easily followed by determining the amount
of salt unchanged in a sample taken at a given moment,[25] the oxidising
reaction of potassium permanganate, in an acid liquid, was employed by
Harcourt and Esson (1865) as one of the first cases for the investigation
of the laws of the rate of chemical change[26] as a subject of great importance
in chemical mechanics. In their experiments they took oxalic acid,[315]
C2H2O_4, which in oxidising gives carbonic anhydride, whilst, with
an excess of sulphuric acid, the potassium permanganate is converted
into manganous sulphate, MnSO4, so that the ultimate oxidation
will be expressed by the equation: 5C2H2O4 + 2MnKO4 + 3H2SO4
= 10CO2 + K2SO4 + 2MnSO4 + 8H2O. The influence of the relative
amount of sulphuric acid is seen from the annexed table, which gives
the measure of reaction p per 100 parts of potassium permanganate,
taken four minutes after mixing, using n molecules of sulphuric acid,
H2SO4, per 2KMnO4 + 5C2H2O4:
| n = |
2 |
4 |
6 |
8 |
12 |
16 |
22 |
| p = |
22 |
36 |
51 |
63 |
77 |
86 |
22 |
showing that in a given time (4 minutes) the oxidation is the more
perfect the greater the amount of sulphuric acid taken for given amounts
of KMnO4 and C2H2O4. It is obvious also that the temperature and
relative amount of every one of the acting and resulting substances
should show its influence on the relative velocity of reaction; thus, for
instance, direct experiment showed the influence of the admixture
of manganous sulphate. When a large proportion of oxalic acid (108
molecules) was taken to a large mass of water and to 2 molecules of
permanganate 14 molecules of manganous sulphate were added, the
quantity x of the potassium permanganate acted on (in percentages
of the potassium permanganate taken) in t minutes (at 16°) was as
follows:
| t = |
2 |
5 |
8 |
11 |
14 |
44 |
47 |
53 |
61 |
68 |
| x = |
5·2 |
12·1 |
18·7 |
25·1 |
31·3 |
68·4 |
71·7 |
75·8 |
79·8 |
83·0 |
[316]
These figures show that the rate of reaction—that is, the quantity of
permanganate changed in one minute—decreases proportionally to the
decrease in the amount of unchanged potassium permanganate. At the
commencement, about 2·6 per cent. of the salt taken was decomposed in
the course of one minute, whilst after an hour the rate was about
0·5 per cent. The same phenomena are observed in every case which
has been investigated, and this branch of theoretical or physical
chemistry, now studied by many,[27] promises to explain the course of
chemical transformations from a fresh point of view, which is closely
allied to the doctrine of affinity, because the rate of reaction, without
doubt, is connected with the magnitude of the affinities acting between
the reacting substances.
[317]
CHAPTER XXII
IRON, COBALT, AND NICKEL
Judging from the atomic weights, and the forms of the higher oxides
of the elements already considered, it is easy to form an idea of
the seven groups of the periodic system. Such are, for instance, the
typical series Li, Be, B, C, N, O, F, or the third series, Na, Mg, Al, Si,
P, S, Cl. The seven usual types of oxides from R2O to R2O7 correspond
with them (Chapter XV.) The position of the eighth group is quite
separate, and is determined by the fact that, as we have already seen,
in each group of metals having a greater atomic weight than potassium
a distinction ought to be made between the elements of the even and
uneven series. The series of even elements, commencing with a
strikingly alkaline element (potassium, rubidium, cæsium), together with
the uneven series following it, and concluding with a haloid (chlorine,
bromine, iodine), forms a large period, the properties of whose members
repeat themselves in other similar periods. The elements of the eighth
group are situated between the elements of the even series and the elements
of the uneven series following them. And for this reason elements
of the eighth group are found in the middle of each large period. The
properties of the elements belonging to it, in many respects independent
and striking, are shown with typical clearness in the case of iron, the
well-known representative of this group.
Iron is one of those elements which are not only widely diffused in
the crust of the earth, but also throughout the entire universe. Its
oxides and their various compounds are found in the most diverse
portions of the earth's crust; but here iron is always found combined
with some other element. Iron is not found on the earth's surface in
a free state, because it easily oxidises under the action of air. It is
occasionally found in the native state in meteorites, or aerolites, which
fall upon the earth.
Meteoric iron is formed outside the earth.[1] Meteorites are fragments
which are carried round the sun in orbits, and fall upon the earth[318]
when coming into proximity with it during their motion in space. The
meteoric dust, on passing through the upper parts of the atmosphere,
and becoming incandescent from friction with the gases, produces that
phenomenon which is familiar under the name of falling stars.[2] Such is[319]
the doctrine concerning meteorites, and therefore the fact of their
containing rocky (siliceous) matter and metallic iron shows that outside
the earth the elements and their aggregation are in some degree the
same as upon the earth itself.
The most widely diffused terrestrial compound of iron is iron
bisulphide, FeS2, or iron pyrites. It occurs in formations of both
aqueous and igneous origin, and sometimes in enormous masses. It is
a substance having a greyish-yellow colour, with a metallic lustre, and a
specific gravity of 5·0; it crystallises in the regular system.[2 bis]
The oxides are the principal ores used for producing metallic iron.
The majority of the ores contain ferric oxide, Fe2O3, either in a
free state or combined with water, or else in combination with ferrous
oxide, FeO. The species and varieties of iron ores are numerous and
diverse. Ferric oxide in a separate form appears sometimes as crystals
of the rhombohedric system, having a metallic lustre and greyish steel
colour; they are brittle, and form a red powder, specific gravity about
5·25. Ferric oxide in type of oxidation and properties resembles
alumina; it is, however, although with difficulty, soluble in acids even
when anhydrous. The crystalline oxide bears the name of specular
iron ore, but ferric oxide most often occurs in a non-crystalline form,
in masses having a red fracture, and is then known as red hæmatite.
In this form, however, it is rather a rare ore, and is principally found
in veins. The hydrates of ferric oxide, ferric hydroxides,[3] are most[320]
often found in aqueous or stratified formations, and are known as
brown hæmatites; they generally have a brown colour, form a yellowish-brown
powder, and have no metallic lustre but an earthy appearance.
They easily dissolve in acids and diffuse through other formations, especially
clays (for instance, ochre); they sometimes occur in reniform and
similar masses, evidently of aqueous origin. Such are, for instance,
the so-called bog or lake and peat ores found at the bottom of marshes
and lakes, and also under and in peat beds. This ore is formed from
water containing ferrous carbonate in solution, which, after absorbing
oxygen, deposits ferric hydroxide. In rivers and springs, iron is found
in solution as ferrous carbonate through the agency of carbonic
acid: hence the existence of chalybeate springs containing FeCO3.
This ferrous carbonate, or siderite, is either found as a non-crystalline
product of evidently aqueous origin, or as a crystalline spar called
spathic iron ore. The reniform deposits of the former are most remarkable;
they are called spherosiderites, and sometimes form whole
strata in the jurassic and carboniferous formations. Magnetic
iron ore, Fe3O4 = FeO,Fe2O3, in virtue of its purity and practical
uses, is a very important ore; it is a compound of the ferrous and
ferric oxides, is naturally magnetic, has a specific gravity of 5·1,
crystallises in well-formed crystals of the regular system, is with difficulty
soluble in acids, and sometimes forms enormous masses, as, for
instance, Mount Blagodat in the Ural. However, in most cases—for
instance, at Korsak-Mogila (to the north of Berdiansk and Nogaiska,
near the Sea of Azov), or at Krivoi Rog (to the west of Ekaterinoslav)—the
magnetic iron ore is mixed with other iron ores. In the Urals, the
Caucasus (without mentioning Siberia), and in the districts adjoining the
basin of the Don, Russia possesses the richest iron ores in the world.
To the south of Moscow, in the Governments of Toula and Nijninovgorod,
in the Olonetz district, and in the Government of Orloffsky
(near Zinovieff in the district of Kromsky), and in many other places,
there are likewise abundant supplies of iron ores amongst the deposited
aqueous formations; the siderite of Orloffsky, for instance, is distinguished
by its great purity.[4]
[321]
Iron is also found in the form of various other compounds—for
instance, in certain silicates, and also in some phosphates; but these
forms are comparatively rare in nature in a pure state, and have not
the industrial importance of those natural compounds of iron previously
mentioned. In small quantities iron enters into the composition
of every kind of soil and all rocky formations. As ferrous oxide,
FeO, is isomorphous with magnesia, and ferric oxide, Fe2O3, with
alumina, isomorphous substitution is possible here, and hence minerals
are not unfrequently found in which the quantity of iron varies considerably;
such, for instance, are pyroxene, amphibole, certain varieties
of mica, &c. Although much iron oxide is deleterious to the growth of
vegetation, still plants do not flourish without iron; it enters as an
indispensable component into the composition of all higher organisms;
in the ash of plants we always find more or less of its compounds. It
also occurs in blood, and forms one of the colouring matters in it;
100 parts of the blood of the highest organisms contain about 0·05 of iron.
The reduction of the ores of iron into metallic iron is in principle
very simple, because when the oxides of iron are strongly heated
with charcoal, hydrogen, carbonic oxide, and other reducing agents,[5]
they easily give metallic iron. But the matter is rendered more[322]
difficult by the fact that the iron does not melt at the heat developed
by the combustion of the charcoal, and therefore it does not separate
from those mechanically mixed impurities which are found in the iron
ore. This is obviated by the following very remarkable property of
iron: at a high temperature it is capable of combining with a small
quantity (from 2 to 5 p.c.) of carbon, and then forms cast iron, which
easily melts in the heat developed by the combustion of charcoal in air.
For this reason metallic iron is not obtained directly from the ore, but
is only formed after the further treatment of the cast iron; the first
product extracted from the ore being cast iron. The fused mass disposes
itself in the furnace below the slag—that is, the impurities of the
ore fused by the heat of the furnace. If these impurities did not fuse
they would block up the furnace in which the ore was being smelted,
and the continuous smelting of the cast iron would not be possible;[6]
it would be necessary periodically to cool the furnace and heat it up
again, which means a wasteful expenditure of fuel, and hence in the
production of cast iron, the object in view is to obtain all the earthy
impurities of the ore in the shape of a fused mass or slag. Only
in rare cases does the ore itself form a mass which fuses at the
temperature employed, and these cases are objectionable if much iron
oxide is carried away in the slag. The impurities of the ores most
often consist of certain mixtures—for instance, a mixture of clay and
sand, or a mixture of limestone and clay, or quartz, &c. These[323]
impurities do not separate of themselves, or do not fuse. The difficulty
of the industry lies in forming an easily-fusible slag, into which the
whole of the foreign matter of the ore would pass and flow down to the
bottom of the furnace above the heavier cast iron. This is effected by
mixing certain fluxes with the ore and charcoal. A flux is a substance
which, when mixed with the foreign matter of the ore, forms a fusible
vitreous mass or slag. The flux used for silica is limestone with clay;
for limestone a definite quantity of silica is used, the best procedure
having been arrived at by experiment and by long practice in iron
smelting and other metallurgical processes.[7]
Thus the following materials have to be introduced into the furnace
where the smelting of the iron ore is carried on: (1) the iron ore,
composed of oxide of iron and foreign matter; (2) the flux required to
form a fusible slag with the foreign matter; (3) the carbon which is
necessary (a) for reducing, (b) for combining with the reduced iron
to form cast iron, (c) principally for the purpose of combustion and
the heat generated thereby, necessary not only for reducing the iron
and transforming it into cast iron, but also for melting the slag, as well
as the cast iron—and (4) the air necessary for the combustion of the
charcoal. The air is introduced after a preparatory heating in order to
economise fuel and to obtain the highest temperature. The air is
forced in under pressure by means of a special blast arrangement.
This permits of an exact regulation of the heat and rate of smelting.
All these component parts necessary for the smelting of iron must be
contained in a vertical, that is, shaft furnace, which at the base must
have a receptacle for the accumulation of the slag and cast iron formed,
in order that the operation may proceed without interruption. The
walls of such a furnace ought to be built of fireproof materials if it be[324]
designed to serve for the continuous production of cast iron by charging
the ore, fuel, and flux into the mouth of the furnace, forcing a blast of
air into the lower part, and running out the molten iron and slag from
below. The whole operation is conducted in furnaces known as blast
furnaces. The annexed illustration, fig. 93 (which is taken by kind
permission from Thorpe's Dictionary of Applied Chemistry), represents
the vertical section of such a furnace. These furnaces are generally
of large dimensions—varying from 50 to 90 feet in height. They are
sometimes built against rising ground in order to afford easy access to
the top where the ore, flux, and charcoal or coke are charged.[8]
[325]
[326]
The cast iron formed in blast furnaces is not always of the same
quality. When slowly cooled it is soft, has a grey colour, and is not
completely soluble in acids. When treated with acids a residue of
graphite remains; it is known as grey or soft cast iron. This is the
general form of the ordinary cast iron used for casting various objects,
because in this state it is not so brittle as in the shape of white cast
iron, which does not leave particles of graphite when dissolved, but
yields its carbon in the form of hydrocarbons. This white cast iron
is characterised by its whitish-grey colour, dull lustre, the crystalline
structure of its fracture (more homogeneous than that of grey iron), and
such hardness that a file will hardly cut it. When white cast iron is
produced (from manganese ore) at high temperatures (and with an excess
of lime), and containing little sulphur and silica but a considerable
amount of carbon (as much as 5 p.c.), it acquires a coarse crystalline
structure which increases in proportion to the amount of manganese,
and it is then known under the name of ‘spiegeleisen’ (and ‘ferro-manganese’).[9]
[327]
Cast iron is a material which is either suitable for direct application
for casting in moulds or else for working up into wrought iron and
steel. The latter principally differ from cast iron in their containing
less carbon—thus, steel contains from 1 p.c. to 0·5 p.c. of carbon and
far less silicon and manganese than cast iron; wrought iron does
not generally contain more than 0·25 p.c. of carbon and not more than
0·25 p.c. of the other impurities. Thus the essence of the working up
of cast iron into steel and wrought iron consists in the removal of the
greater part of the carbon and other elements, S, P, Mn, Si, &c. This
is effected by means of oxidation, because the oxygen of the atmosphere,
oxidising the iron at a high temperature, forms solid oxides with it;
and the latter, coming into contact with the carbon contained in the
cast iron, are deoxidised, forming wrought iron and carbonic oxide,
which is evolved from the mass in a gaseous form. It is evident that
the oxidation must be carried on with a molten mass in a state of
agitation, so that the oxygen of the air may be brought into contact
with the whole mass of carbon contained in the cast iron, or else the
operation is effected by means of the addition of oxygen compounds
of iron (oxides, ores, as in Martin's process). Cast iron melts much
more easily than wrought iron and steel, and, therefore, as the carbon
separates, the mass in the furnace (in puddling) or hearth (in the
bloomery process) becomes more and more solid; moreover the degree of
hardness forms, to a certain extent, a measure of the amount of carbon
separated, and the operation may terminate either in the formation of
steel or wrought iron.[10] In any case, the iron used for industrial purposes[328]
contains impurities. Chemically pure iron may be obtained by
precipitating iron from a solution (a mixture of ferrous sulphate with[329]
magnesium sulphate or ammonium chloride) by the prolonged action of
a feeble galvanic current; the iron may be then obtained as a dense[330]
[331]
[332]
mass. This method, proposed by Böttcher and applied by Klein, gives,
as R. Lenz showed, iron containing occluded hydrogen, which is disengaged
on heating. This galvanic deposition of iron is used for
making galvanoplastic clichés, which are distinguished for their great
hardness. Electro-deposited iron is brittle, but if heated (after the
separation of the hydrogen) it becomes soft. If pure ferric hydroxide,
which is easily prepared by the precipitation of solutions of ferric
salts by means of ammonia, be heated in a stream of hydrogen, it
forms, first of all, a dull black powder which ignites spontaneously in
air (pyrophoric iron), and then a grey powder of pure iron. The
powdery substance first obtained is an iron suboxide; when thrown
into the air it ignites, forming the oxide Fe3O4. If the heating in
hydrogen be continued, more water and pure iron, which does not
ignite spontaneously, will be obtained. If a small quantity of iron be
fused in the oxyhydrogen flame (with an excess of oxygen) in a piece
of lime and mixed with powdered glass, pure molten iron will be
formed, because in the oxyhydrogen flame iron melts and burns, but
the substances mixed with the iron oxidise first. The oxidised impurities
here either disappear (carbonic anhydride) in a gaseous form,
or turn into slag (silica, manganese, oxide, and others)—that is, fuse
with the glass. Pure iron has a silvery white colour and a specific
gravity of 7·84; it melts at a temperature higher than the melting-points
of silver, gold, nickel, and steel, i.e. about 1400°-1500° and[333]
below the melting point of platinum (1750°).[11] But pure iron becomes
soft at a temperature considerably below that at which it melts, and
may then be easily forged, welded, and rolled or drawn into sheets and
wire.[11 bis] Pure iron may be rolled into an exceedingly thin sheet,
weighing less than a sheet of ordinary paper of the same size. This
ductility is the most important property of iron in all its forms, and is
most marked with sheet iron, and least so with cast iron, whose
ductility, compared with wrought iron, is small, but it is still very
considerable when compared with other substances—such, for instance,
as rocks.[12]
The chemical properties of iron have been already repeatedly
mentioned in preceding chapters. Iron rusts in air at the ordinary
temperature—that is to say, it becomes covered with a layer of iron
oxides. Here, without doubt, the moisture of the air plays a part,
because in dry air iron does not oxidise at all, and also because, more[334]
particularly, ammonia is always found in iron rust; the ammonia must
arise from the action of the hydrogen of the water, at the moment of its
separation, on the nitrogen of the air. Highly-polished steel does not
rust nearly so readily, but if moistened with water, it easily becomes
coated with rust. As rust depends on the access of moisture, iron may
be preserved from rust by coating it with substances which prevent
the moisture having access to it. Thus arises the practice of covering
iron objects with paraffin,[13] varnish, oil, paints, or enamelling it
with a glassy-looking flux possessing the same coefficient of expansion as
iron, or with a dense scoria (formed by the heat of superheated steam),
or with a compact coating of various metals. Wrought iron (both as
sheet iron and in other forms), cast iron, and steel are often coated with
tin, copper, lead, nickel, and similar metals, which prevent contact with
the air. These metals preserve iron very effectually from rust if they
form a completely compact surface, but in those places where the iron
becomes exposed, either accidentally or from wear, rust appears much
more quickly than on a uniform iron surface, because, towards these
metals (and also towards the rust), the iron will then behave as an
electro-positive pole in a galvanic couple, and hence will attract
oxygen. A coating of zinc does not produce this inconvenience, because
iron is electro-negative with reference to zinc, in consequence of which
galvanised iron does not easily rust, and even an iron boiler containing
some lumps of zinc rusts less than one without zinc.[14] Iron oxidises
at a high temperature, forming iron scale, Fe3O4, composed of ferrous
and ferric oxides, and, as has been seen, decomposes water and acids
with the evolution of hydrogen. It is also capable of decomposing
salts and oxides of other metals, which property is applied in the arts
for the extraction of copper, silver, lead, tin, &c. For this reason
iron is soluble in the solutions of many salts—for instance, in cupric
sulphate, with precipitation of copper and formation of ferrous sulphate.[15]
When iron acts on acids it always forms compounds FeX2—that[335]
is, corresponding to the suboxide FeO—and answering to magnesium
compounds—and hence two atoms of hydrogen are replaced by one
atom of iron. Strongly oxidising acids like nitric acid may transform
the ferrous salt which is forming into the higher degree of oxidation or
ferric salt (corresponding with the sesquioxide, Fe2O3), but this is a
secondary reaction. Iron, although easily soluble in dilute nitric acid,
loses this property when plunged into strong fuming nitric acid; after
this operation it even loses the property of solubility in other acids
until the external coating formed by the action of the strong nitric
acid is mechanically removed. This condition of iron is termed the
passive state. The passive condition of iron depends on the formation,
on its surface, of a coating of oxide due to the iron being acted on by
the lower oxides of nitrogen contained in the fuming nitric acid.[16]
Strong nitric acid which does not contain these lower oxides, does not
render iron passive, but it is only necessary to add some alcohol or
other reducing agent which forms these lower oxides in the nitric acid,
and the iron will assume the passive state.
Iron readily combines with non-metals—for instance, with chlorine,
iodine, bromine, sulphur, and even with phosphorus and carbon; but
on the other hand the property of combining with metals is but little
developed in it—that is to say, it does not easily form alloys. Mercury,
which acts on most metals, does not act directly on iron, and the iron
amalgam, or solution of iron in mercury, which is used for electrical
machines, is only obtained in a particular way—namely, with the
co-operation of a sodium amalgam, in which the iron dissolves and by
means of which it is reduced from solutions of its salts.
When iron acts on acids it forms ferrous salts of the type FeX2,
and in the presence of air and oxidising agents they change by degrees
into ferric salts of the type FeX3. This faculty of passing from the
ferrous to the ferric state is still further developed in ferrous hydroxide.
If sodium hydroxide be added to a solution of ferrous sulphate or
green vitriol, FeSO4,[17] a white precipitate of ferrous hydroxide, FeH2O2,[336]
is obtained; but on exposure to the air, even under water, it turns
green, becomes grey, and finally turns brown, which is due to the
oxidation that it undergoes. Ferrous hydroxide is very sparingly
soluble in water; the solution has, however, a distinct alkaline reaction,
which is due to its being a fairly energetic basic oxide. In any case,
ferrous oxide is far more energetic than ferric oxide, so that if ammonia
be added to a solution containing a mixture of a ferrous and ferric
salt, at first ferric hydroxide only will be precipitated. If barium
carbonate, BaCO3, be shaken up in the cold with ferrous salts, it
does not precipitate them—that is, does not change them into ferrous
carbonate; but it completely separates all the iron from the ferric
salts in the cold, according to the equation Fe2Cl6 + 3BaCO3 + 3H2O
= Fe2O3,3H2O + 3BaCl2 + 3CO2. If ferrous hydroxide be boiled with
a solution of potash, the water is decomposed, hydrogen is evolved, and
the ferrous hydroxide is oxidised. The ferrous salts are in all respects
similar to the salts of magnesium and zinc; they are isomorphous
with them, but differ from them in that the ferrous hydroxide is not
soluble either in aqueous potash or ammonia. In the presence of an
excess of ammonium salts, however, a certain proportion of the iron[337]
is not precipitated by alkalis and alkali carbonates, which fact points
to the formation of double ammonium salts.[18] The ferrous salts have
a dull greenish colour, and form solutions also of a pale green colour,
whilst the ferric salts have a brown or reddish-brown colour. The
ferrous salts, being capable of oxidation, form very active reducing
agents—for instance, under their action gold chloride, AuCl3, deposits
metallic gold, nitric acid is transformed into lower oxides, and the
highest oxides of manganese also pass into the lower forms of oxidation.
All these reactions take place with especial ease in the presence of an
excess of acid. This depends on the fact that the ferrous oxide, FeO
(or salt), acting as a reducing agent, turns into ferric oxide, Fe2O3 (or
salt), and in the ferric state it requires more acid for the formation
of a normal salt than in the ferrous condition. Thus in the normal
ferrous sulphate, FeSO4, there is one equivalent of iron to one
equivalent of sulphur (in the sulphuric radicle), but in the neutral
ferric salt, Fe2(SO4)3, there is one equivalent of iron to one and a
half of sulphur in the form of the elements of sulphuric acid.[19]
The most simple oxidising agent for transforming ferrous into ferric
salts is chlorine in the presence of water—for instance, 2FeCl2 + Cl2[338]
= Fe2Cl6, or, generally speaking, 2FeO + Cl2 + H2O = Fe2O3 + 2HCl.
When such a transformation is required it is best to add potassium
chlorate and hydrochloric acid to the ferrous solution; chlorine is
formed by their mutual reaction and acts as an oxidising agent.
Nitric acid produces a similar effect, although more slowly. Ferrous
salts may be completely and rapidly oxidised into ferric salts by means
of chromic acid or permanganic acid, HMnO4, in the presence of acids—for
example, 10FeSO4 + 2KMnO4 + 8H2SO4 = 5Fe2(SO4)3 + 2MnSO4
+ K2SO4 + 8H2O. This reaction is easily observed by the change of
colour, and its termination is easily seen, because potassium permanganate
forms solutions of a bright red colour, and when added to a
solution of a ferrous salt the above reaction immediately takes place in
the presence of acid, and the solution then becomes colourless, because all
the substances formed are only faintly coloured in solution. Directly all
the ferrous compound has passed into the ferric state, any excess of
permanganate which is added communicates a red colour to the liquid
(see Chapter XXI.)
Thus when ferrous salts are acted on by oxidising agents, they pass
into the ferric form, and under the action of reducing agents the
reverse reaction occurs. Sulphuretted hydrogen may, for instance, be
used for this complete transformation, for under its influence ferric salts
are reduced with separation of sulphur—for example, Fe2Cl6 + H2S
= 2FeCl2 + 2HCl + S. Sodium thiosulphate acts in a similar way:
Fe2Cl6 + Na2S2O3 + H2O = 2FeCl2 + Na2SO4 + 2HCl + S. Metallic
iron or zinc,[20] in the presence, of acids, or sodium amalgam, &c.,
acts like hydrogen, and has also a similar reducing action, and this
furnishes the best method for reducing ferric salts to ferrous salts—for
instance, Fe2Cl6 + Zn = 2FeCl2 + ZnCl2. Thus the transition from
ferrous salts to ferric salts and vice versâ is always possible.[21]
[339]
Ferric oxide, or sesquioxide of iron, Fe2O3, is found in nature,
and is artificially prepared in the form of a red powder by many
methods. Thus after heating green vitriol a red oxide of iron remains,
called colcothar, which is used as an oil paint, principally for painting
wood. The same substance in the form of a very fine powder (rouge)
is used for polishing glass, steel, and other objects. If a mixture of
ferrous sulphate with an excess of common salt be strongly heated,
crystalline ferric oxide will be formed, having a dark violet colour, and
resembling some natural varieties of this substance. When iron pyrites
is heated for preparing sulphurous anhydride, ferric oxide also remains
behind; it is used as a pigment. On the addition of alkalis to a
solution of ferric salts, a brown precipitate of ferric hydroxide is formed,
which when heated (even when boiled in water, that is, at about 100°,
according to Tomassi) easily parts with the water, and leaves red
anhydrous ferric oxide. Pure ferric oxide does not show any magnetic
properties, but when heated to a white heat it loses oxygen and is
converted into the magnetic oxide. Anhydrous ferric oxide which has
been heated to a high temperature is with difficulty soluble in acids
(but it is soluble when heated in strong acids, and also when fused with
potassium hydrogen sulphate), whilst ferric hydroxide, at all events
that which is precipitated from salts by means of alkalis, is very readily
soluble in acids. The precipitated ferric hydroxide has the composition
2Fe2O33H2O, or Fe4H6O9. If this ordinary hydroxide be rendered
anhydrous (at 100°), at a certain moment it becomes incandescent—that
is, loses a certain quantity of heat. This self-incandescence
depends on internal displacement produced by the transition of the
easily-soluble (in acids) variety into the difficultly-soluble variety,
and does not depend on the loss of water, since the anhydrous oxide
undergoes the same change. In addition to this there exists a ferric
hydroxide, or hydrated oxide of iron, which, like the strongly-heated
anhydrous iron oxide, is difficultly soluble in acids. This hydroxide on
losing water, or after the loss of water, does not undergo such self-incandescence,
because no such state of internal displacement occurs
(loss of energy or heat) with it as that which is peculiar to the ordinary
oxide of iron. The ferric hydroxide which is difficultly soluble in acids
has the composition Fe2O3,H2O. This hydroxide is obtained by a prolonged[340]
ebullition of water in which ferric hydroxide prepared by the
oxidation of ferrous oxide is suspended, and also sometimes by similar
treatment of the ordinary hydroxide after it has been for a long time
in contact with water. The transition of one hydroxide to another is
apparent by a change of colour; the easily-soluble hydroxide is redder,
and the sparingly-soluble hydroxide more yellow in colour.[22]
The normal salts of the composition Fe2X6 or FeX3 correspond
with ferric oxide—for example, the exceedingly volatile ferric chloride,
Fe2Cl6, which is easily prepared in the anhydrous state by the action
of chlorine on heated iron.[23] Such also is the normal ferric nitrate,[341]
[342]
Fe2(NO3)6; it is obtained by dissolving iron in an excess of nitric acid,
taking care as far as possible to prevent any rise of temperature.[24] The
normal salt separates from the brown solution when it is concentrated[343]
under a bell jar over sulphuric acid. This salt, Fe2(NO3)6,9H2O, then
crystallises in well-formed and perfectly colourless crystals,[25] which
deliquesce in air, melt at 35°, and are soluble in and decomposed by
water. The decomposition may be seen from the fact that the solution
is brown and does not yield the whole of the salt again, but gives
partly basic salt. The normal salt (only stable in the presence of an
excess of HNO3) is completely decomposed with great facility by heating
with water, even at 130°, and this is made use of for removing iron
(and also certain other oxides of the form R2O3) from many other
bases (of the form RO) whose nitrates are far more stable. The ferric
salts, FeX3, in passing into ferrous salts, act as oxidising agents, as is
seen from the fact that they not only liberate S from SH2, but also
iodine from KI like many oxidising agents.[25 bis]
[344]
Iron forms one other oxide besides the ferric and ferrous oxides;
this contains twice as much oxygen as the former, but is so very
unstable that it can neither be obtained in the free state nor as a
hydrate. Whenever such conditions of double decomposition occur as
should allow of its separation in the free state, it decomposes into
oxygen and ferric oxide. It is known in the state of salts, and is only
stable in the presence of alkalis, and forms salts with them which have
a decidedly alkaline reaction; it is therefore a feebly acid oxide. Thus
when small pieces of iron are heated with nitre or potassium chlorate
a potassium salt of the composition K2FeO4 is formed, and therefore
the hydrate corresponding with this salt should have the composition
H2FeO4. It is called ferric acid. Its anhydride ought to contain
FeO3 or Fe2O6—twice as much oxygen as ferric oxide. If a solution
of potassium ferrate be mixed with acid, the free hydrate ought
to be formed, but it immediately decomposes (2K2FeO4 + 5H2SO4
= 2K2SO4 + Fe2(SO4)3 + 5H2O + O3), oxygen being evolved. If a
small quantity of acid be taken, or if a solution of potassium ferrate
be heated with solutions of other metallic salts, ferric oxide is separated—for
instance:
2CuSO4 + 2K2FeO4 = 2K2SO4 + O3 + Fe2O3 + 2CuO.
Both these oxides are of course deposited in the form of hydrates.
This shows that not only the hydrate H2FeO4, but also the salts of the
heavy metals corresponding with this higher oxide of iron, are not
formed by reactions of double decomposition. The solution of potassium
ferrate naturally acts as a powerful oxidising agent; for instance,
it transforms manganous oxide into the dioxide, sulphurous into
sulphuric acid, oxalic acid into carbonic anhydride and water, &c.[26]
Iron thus combines with oxygen in three proportions: RO, R2O3,[345]
and RO3. It might have been expected that there would be intermediate
stages RO2 (corresponding to pyrites FeS2) and R2O5, but for
iron these are unknown.[26 bis] The lower oxide has a distinctly basic
character, the higher is feebly acid. The only one which is stable in
the free state is ferric oxide, Fe2O3; the suboxide, FeO, absorbs
oxygen, and ferric anhydride, FeO3, evolves it. It is also the same
for other elements; the character of each is determined by the relative
degree of stability of the known oxides. The salts FeX2 correspond
with the suboxide, the salts FeX3 or Fe2X6 with the sesquioxide, and
FeX6 represents those of ferric acid, as its potassium salt is FeO2(OK)2,
corresponding with K2SO4, K2MnO4, K2CrO4, &c. Iron therefore
forms compounds of the types FeX2, FeX3, and FeX6, but this latter,
like the type NX5, does not appear separately, but only when X represents
heterogeneous elements or groups; for instance, for nitrogen
in the form of NO2(OH), NH4Cl, &c., for iron in the form of
FeO2(OK)2. But still the type FeX6 exists, and therefore FeX2 and
FeX3 are compounds which, like ammonia, NH3, are capable of further
combinations up to FeX6; this is also seen in the property of
ferrous and ferric salts of forming compounds with water of crystallisation,
besides double and basic salts, whose stability is determined by
the quality of the elements included in the types FeX2 and FeX3.[26 tri]
It is therefore to be expected that there should be complex compounds[346]
derived from ferrous and ferric oxides. Amongst these the series
of cyanogen compounds is particularly interesting; their formation
and character is not only determined by the property which iron
possesses of forming complex types, but also by the similar faculty of
the cyanogen compounds, which, like nitriles (Chapter IX.), have
clearly developed properties of polymerisation and in general of forming
complex compounds.[27]
In the cyanogen compounds of iron, two degrees might be expected:
Fe(CN)2, corresponding with ferrous oxide, and Fe(CN)3, corresponding
with ferric oxide. There are actually, however, many other known
compounds, intermediate and far more complex. They correspond
with the double salts so easily formed by metallic cyanides. The two
following double salts are particularly well known, very stable, often
used, and easily prepared. Potassium ferrocyanide or yellow prussiate
of potash, a double salt of cyanide of potassium and ferrous cyanide,
has the composition FeC2N2,4KCN; its crystals contain 3 mol. of water:
K4FeC6N6,3H2O. The other is potassium ferricyanide or red prussiate
of potash. It is also known as Gmelin's salt, and contains cyanide of
potassium with ferric cyanide; its composition is Fe(CN)3,3KCN or
K3FeC6N6. Its crystals do not contain water. It is obtained from
the first by the action of chlorine, which removes one atom of the
potassium. A whole series of other ferrocyanic compounds correspond
with these ordinary salts.
Before treating of the preparation and properties of these two
remarkable and very stable salts, it must be observed that with ordinary
reagents neither of them gives the same double decompositions
as the other ferrous and ferric salts, and they both present a series of
remarkable properties. Thus these salts have a neutral reaction, are
unchanged by air, dilute acids, or water, unlike potassium cyanide and
even some of its double salts. When solutions of these salts are treated
with caustic alkalis, they do not give a precipitate of ferrous or ferric
hydroxides, neither are they precipitated by sodium carbonate. This
led the earlier investigators to recognise special independent groupings
in them. The yellow prussiate was considered to contain the complex
radicle FeC6N6 combined with potassium, namely with K4, and K3
was attributed to the red prussiate. This was confirmed by the fact
that whilst in both salts any other metal, even hydrogen, might be
substituted for potassium, the iron remained unchangeable, just as
nitrogen in cyanogen, ammonium, and nitrates does not enter into[347]
double decomposition, being in the state of the complex radicles CN,
NH4, NO2. Such a representation is, however, completely superfluous
for the explanation of the peculiarities in the reactions of such compounds
as double salts. If a magnesium salt which can be precipitated
by potassium hydroxide does not form a precipitate in the presence of
ammonium chloride, it is very clear that it is owing to the formation
of a soluble double salt which is not decomposed by alkalis. And
there is no necessity to account for the peculiarity of reaction of a
double salt by the formation of a new complex radicle. In the same
way also, in the presence of an excess of tartaric acid, cupric salts do
not form a precipitate with potassium hydroxide, because a double salt
is formed. These peculiarities are more easily understood in the case
of cyanogen compounds than in all others, because all cyanogen compounds,
as unsaturated compounds, show a marked tendency to
complexity. This tendency is satisfied in double salts. The appearance
of a peculiar character in double cyanides is the more easily
understood since in the case of potassium cyanide itself, and also in
hydrocyanic acid, a great many peculiarities have been observed
which are not encountered in those haloid compounds, potassium
chloride and hydrochloric acid, with which it was usual to compare
cyanogen compounds. These peculiarities become more comprehensible
on comparing cyanogen compounds with ammonium compounds. Thus
in the presence of ammonia the reactions of many compounds change
considerably. If in addition to this it is remembered that the
presence of many carbon (organic) compounds frequently completely
disturbs the reaction of salts, the peculiarities of certain double cyanides
will appear still less strange, because they contain carbon. The fact
that the presence of carbon or another element in the compound produces
a change in the reactions, may be compared to the action of
oxygen, which, when entering into a combination, also very materially
changes the nature of reactions. Chlorine is not detected by silver
nitrate when it is in the form of potassium chlorate, KClO3, as it is
detected in potassium chloride, KCl. The iron in ferrous and ferric
compounds varies in its reactions. In addition to the above-mentioned
facts, consideration ought to be given to the circumstance that the
easy mutability of nitric acid undergoes modification in its alkali
salts, and in general the properties of a salt often differ much from
those of the acid. Every double salt ought to be regarded as a peculiar
kind of saline compound: potassium cyanide is, as it were, a basic,
and ferrous cyanide an acid, element. They may be unstable in the
separate state, but form a stable double compound when combined
together; the act of combination disengages the energy of the elements,[348]
and they, so to speak, saturate each other. Of course, all this is not a
definite explanation, but then the supposition of a special complex radicle
can even less be regarded as such.
Potassium ferrocyanide, K4FeC6N6, is very easily formed by mixing
solutions of ferrous sulphate and potassium cyanide. First, a white
precipitate of ferrous cyanide, FeC2N2, is formed, which becomes blue
on exposure to air, but is soluble in an excess of potassium cyanide,
forming the ferrocyanide. The same yellow prussiate is obtained on
heating animal nitrogenous charcoal or animal matters—such as
horn, leather cuttings, &c.—with potassium carbonate in iron
vessels,[27 bis] the mass formed being afterwards boiled with water with
exposure to air, potassium cyanide first appearing, which gives yellow
prussiate. The animal charcoal may be exchanged for wood charcoal,
permeated with potassium carbonate and heated in nitrogen or
ammonia; the mass thus produced is then boiled in water with ferric
oxide.[28] In this manner it is manufactured on the large scale, and is
called ‘yellow prussiate’ (‘prussiate de potasse,’ Blutlaugensalz).
It is easy to substitute other metals for the potassium in the yellow
prussiate. The hydrogen salt or hydroferrocyanic acid, H4FeC6N6, is
obtained by mixing strong solutions of yellow prussiate and hydrochloric
acid. If ether be added and the air excluded, the acid is
obtained directly in the form of a white scarcely crystalline precipitate
which becomes blue on exposure to air (as ferrous cyanide does from the
formation of blue compounds of ferrous and ferric cyanides, and it is
on this account used in cotton printing). It is soluble in water and
alcohol, but not in ether, has marked acid properties, and decomposes
carbonates, which renders it easily possible to prepare ferrocyanides of[349]
the metals of the alkalis and alkaline earths; these are readily soluble,
have a neutral reaction, and resemble the yellow prussiate. Solutions
of these salts form precipitates with the salts of other metals, because the
ferrocyanides of the heavy metals are insoluble. Here either the whole
of the potassium of the yellow prussiate, or only a part of it, is exchanged
for an equivalent quantity of the heavy metal. Thus, when a cupric
salt is added to a solution of yellow prussiate, a red precipitate is obtained
which still contains half the potassium of the yellow prussiate:
K4FeC6N6 + CuSO4 = K2CuFeC6N6 + K2SO4.
But if the process be reversed (the salt of copper being then in excess)
the whole of the potassium will be exchanged for copper, forming a
reddish-brown precipitate, Cu2FeC6N6,9H2O. This reaction and
those similar to it are very sensitive and may be used for detecting
metals in solution, more especially as the colour of the precipitate
very often shows a marked difference when one metal is exchanged
for another. Zinc, cadmium, lead, antimony, tin, silver, cuprous and
aurous salts form white precipitates; cupric, uranium, titanium
and molybdenum salts reddish-brown; those of nickel, cobalt,
and chromium, green precipitates; with ferrous salts, ferrocyanide
forms, as has been already mentioned, a white precipitate—namely,
Fe2FeC6N6, or FeC2N2—which turns blue on exposure to air, and
with ferric salts a blue precipitate called Prussian blue. Here the
potassium is replaced by iron, the reaction being expressed thus:
2Fe2Cl6 + 3K4FeC6N6 = 12KCl + Fe4Fe3C18N18, the latter formula
expressing the composition of Prussian blue. It is therefore the
compound 4Fe(CN)3 + 3Fe(CN)2. The yellow prussiate is prepared in
chemical works on a large scale especially for the manufacture of this
blue pigment, which is used for dyeing cloth and other fabrics and
also as one of the ordinary blue paints. It is insoluble in water, and
the stuffs are therefore dyed by first soaking them in a solution of a
ferric salt and then in a solution of yellow prussiate. If however
an excess of yellow prussiate be present complete substitution between
potassium and iron does not occur, and soluble Prussian blue is
formed; KFe2(CN)6 = KCN,Fe(CN)2,Fe(CN)3. This blue salt is
colloidal, is soluble in pure water, but insoluble and precipitated
when other salts—for instance, potassium or sodium chloride—are
present even in small quantities, and is therefore first obtained as a
precipitate.[29]
[350]
Potassium ferricyanide, or red prussiate of potash, K3FeC6N6, is
called ‘Gmelin's salt,’ because this savant obtained it by the action
of chlorine on a solution of the yellow prussiate: K4FeC6N6 + Cl
= K3FeC6N6 + KCl. The reaction is due to the ferrous salt being
changed by the action of the chlorine into a ferric salt. It separates
from solutions in anhydrous, well-formed prisms of a red colour, but
the solution has an olive colour; 100 parts of water, at 10°, dissolve
37 parts of the salt, and at 100°, 78 parts.[30] The red prussiate
gives a blue precipitate with ferrous salts, called Turnbull's blue,
very much like Prussian blue (and the soluble variety), because it also
contains ferrous cyanide and ferric cyanide, although in another proportion,[351]
being formed according to the equation: 3FeCl2 + 2K3FeC6N6
= 6KCl + Fe3Fe2C12N12, or 3FeC2N2,Fe2C6N6; in Prussian blue we
have Fe7Cy18, and here Fe5Cy12. A ferric salt ought to form ferric
cyanide Fe2C6N6, with red prussiate, but ferric cyanide is soluble,
and therefore no precipitate is obtained, and the liquid only becomes
brown.[31]
If chlorine and sodium are representatives of independent groups
of elements, the same may also be said of iron. Its nearest analogues
show, besides a similarity in character, a likeness as regards
physical properties and a proximity in atomic weight. Iron occupies a
medium position amongst its nearest analogues, both with respect to
properties and faculty of forming saline oxides, and also as regards
atomic weight. On the one hand, cobalt, 58, and nickel, 59, approach[352]
iron, 56; they are metals of a more basic character, they do not form
stable acids or higher degrees of oxidation, and are a transition to
copper, 63, and zinc, 65. On the other hand, manganese, 55, and
chromium, 52, are the nearest to iron; they form both basic and acid
oxides, and are a transition to the metals possessing acid properties.
In addition to having atomic weights approximately alike, chromium,
manganese, iron, cobalt, nickel, and copper have also nearly the same
specific gravity, so that the atomic volumes and the molecules of their
analogous compounds are also near to one another (see table at the
beginning of this volume). Besides this, the likeness between the
above-mentioned elements is also seen from the following:
They form suboxides, RO, fairly energetic bases, isomorphous with
magnesia—for instance, the salt RSO4,7H2O, akin to MgSO4,7H2O,
and FeSO4,7H2O, or to sulphates containing less water; with alkali
sulphates all form double salts crystallising with 6H2O; all are capable
of forming ammonium salts, &c. The lower oxides, in the cases of
nickel and cobalt, are tolerably stable, are not easily oxidised (the
nickel compound with more difficulty than cobalt, a transition to
copper); with manganese, and especially with chromium, they are
more easily oxidised than with iron and pass into higher oxides.
They also form oxides of the form R2O3, and with nickel, cobalt,
and manganese this oxide is very unstable, and is more easily reduced
than ferric oxide; but, in the case of chromium, it is very stable, and
forms the ordinary kind of salts. It is isomorphous with ferric oxide,
forms alums, is a feeble base, &c. Chromium, manganese, and iron are
oxidised by alkali and oxidising agents, forming salts like Na2SO4;
but cobalt and nickel are difficult to oxidise; their acids are not known
with any certainty, and are, in all probability, still less stable than the
ferrates. Cr, Mn and Fe form compounds R2Cl6 which are like Fe2Cl6
in many respects; in Co this faculty is weaker and in Ni it has almost
disappeared. The cyanogen compounds, especially of manganese and
cobalt, are very near akin to the corresponding ferrocyanides. The
oxides of nickel and cobalt are more easily reduced to metal than those
of iron, but those of manganese and chromium are not reduced so
easily as iron, and the metals themselves are not easily obtained in a
pure state; they are capable of forming varieties resembling cast iron.
The metals Cr, Mn, Fe, Co, and Ni have a grey iron colour and are very
difficult to melt, but nickel and cobalt can be melted in the reverberatory
furnace and are more fusible than iron, whilst chromium is more
difficult to melt than platinum (Deville). These metals decompose
water, but with greater difficulty as the atomic weight rises, forming a
transition to copper, which does not decompose water. All the compounds[353]
of these metals have various colours, which are sometimes very
bright, especially in the higher stages of oxidation.
These metals of the iron group are often met with together in
nature. Manganese nearly everywhere accompanies iron, and iron is
always an ingredient in the ores of manganese. Chromium is found
principally as chrome ironstone—that is, a peculiar kind of magnetic
oxide in which Fe2O3 is replaced by Cr2O3.
Nickel and cobalt are as inseparable companions as iron and
manganese. The similarity between them even extends to such
remote properties as magnetic qualities. In this series of metals we
find those which are the most magnetic: iron, cobalt, and nickel.
There is even a magnetic oxide among the chromium compounds, such
being unknown in the other series. Nickel easily becomes passive in
strong nitric acid. It absorbs hydrogen in just the same way as iron.
In short, in the series Cr, Mn, Fe, Co, and Ni, there are many points
in common although there are many differences, as will be seen still
more clearly on becoming acquainted with cobalt and nickel.
In nature cobalt is principally found in combination with arsenic
and sulphur. Cobalt arsenide, or cobalt speiss, CoAs2, is found in
brilliant crystals of the regular system, principally in Saxony. Cobalt
glance, CoAs2CoS2, resembles it very much, and also belongs to the
regular system; it is found in Sweden, Norway, and the Caucasus.
Kupfernickel is a nickel ore in combination with arsenic, but of a
different composition from cobalt arsenide, having the formula NiAs;
it is found in Bohemia and Saxony. It has a copper-red colour and is
rarely crystalline; it is so called because the miners of Saxony first
mistook it for an ore of copper (Kupfer), but were unable to extract
copper from it. Nickel glance, NiS2,NiAs2, corresponding with cobalt
glance, is also known. Nickel accompanies the ores of cobalt and
cobalt those of nickel, so that both metals are found together. The
ores of cobalt are worked in the Caucasus in the Government of
Elizavetopolsk. Nickel ores containing aqueous hydrated nickel silicate
are found in the Ural (Revdansk). Large quantities of a similar ore
are exported into Europe from New Caledonia. Both ores contain
about 12 per cent. Ni. Garnierite, (RO)5(SiO2)41½H2O, where R = Ni
and Mg, predominates in the New Caledonian ore. Large deposits of
nickel have been discovered in Canada, where the ore (as nickelous
pyrites) is free from arsenic. Cobalt is principally worked up into
cobalt compounds, but nickel is generally reduced to the metallic state, in
which it is now often used for alloys—for instance, for coinage in many
European States, and for plating other metals, because it does not
oxidise. Cobalt arsenide and cobalt glance are principally used for the[354]
preparation of cobalt compounds; they are first sorted by discarding
the rocky matter, and then roasted. During this process most of the
sulphur and arsenic disappears; the arsenious anhydride volatilises
with the sulphurous anhydride and the metal also oxidises.[32] It is a
simple matter to obtain nickel and cobalt from their oxides. In order
to obtain the latter, solutions of their salts are treated with sodium[355]
carbonate and the precipitated carbonates are heated; the suboxides
are thus obtained, and these latter are reduced in a stream of
hydrogen, or even by heating with ammonium chloride. They easily
oxidise when in the state of powder. When the chlorides of nickel
and cobalt are heated in a stream of hydrogen, the metal is deposited
in brilliant scales. Nickel is always much more easily and quickly
reduced than cobalt. Nickel melts more easily than cobalt, and this
even furnishes a means of testing the heating powers of a reverberatory
furnace. Cobalt fuses at a temperature only a little lower than that
at which iron does. In general, cobalt is nearer to iron than nickel,
nickel being nearer to copper.[32 bis] Both nickel and cobalt have magnetic
properties like iron, but Co is less magnetic than Fe, and Ni still
less so. The specific gravity of nickel reduced by hydrogen is 9·1 and
that of cobalt 8·9. Fused cobalt has a specific gravity of 8·5, the
density of ordinary nickel being almost the same. Nickel has a greyish
silvery-white colour; it is brilliant and very ductile, so that the finest
wire may be easily drawn from it. This wire has a resistance to
tension equal to iron wire. The beautiful colour of nickel, and the
high polish which it is capable of receiving and retaining, as it does
not oxidise, render it a useful metal for many purposes, and in
many ways it resembles silver.[32 tri] It is now very common to cover[356]
other metals with a layer of nickel (nickel plating). This is done by a
process of electro-plating, using a solution of a nickel salt. The
colour of cobalt is dark and redder; it is also ductile, and has a
greater tensile resistance than iron. Dilute acids act very slowly on
nickel and cobalt; nitric acid may be considered as the best solvent
for them. The solutions in every case contain salts corresponding with
the ferrous salts—that is, the salts CoX2, NiX2, correspond with the
suboxides of these metals. These salts in their types are similar to the
magnesium salts. The salts of nickel when crystallising with water
have a green colour, and form bright green solutions, but in the anhydrous
state they most frequently have a yellow colour. The salts of
cobalt are generally rose-coloured, and generally blue when in the
anhydrous state. Their aqueous solutions are rose-coloured. Cobaltous
chloride is easily soluble in alcohol, and forms a solution of an intense
blue colour.[33]
[357]
If a solution of potassium hydroxide be added to a solution of a
cobalt salt, a blue precipitate of the basic salt will be formed. If a[358]
solution of a cobalt salt be heated almost to the boiling-point, and the
solution be then mixed with a boiling solution of an alkali hydroxide,
a pink precipitate of cobaltous hydroxide, CoH2O2, will be formed. If
air be not completely excluded during the precipitation by boiling, the
precipitate will also contain brown cobaltic hydroxide formed by the
further oxidation of the cobaltous oxide.[34] Under similar circumstances
nickel salts form a green precipitate of nickelous hydroxide, the formation
of which is not hindered by the presence of ammonium salts, but
in that case only requires more alkali to completely separate the
nickel. The nickelous oxide obtained by heating the hydroxide, or
from the carbonate or nitrate, is a grey powder, easily soluble in acids
and easily reduced, but the same substance may be obtained in the
crystalline form as an ordinary product from the ores; it crystallises
in regular octahedra, with a metallic lustre, and is of a grey colour.
In this state the nickelous oxide almost resists the action of acids.[34 bis]
[359]
It is interesting to note the relation of the cobaltous and nickelous
hydroxides to ammonia; aqueous ammonia dissolves the precipitate of
cobaltous and nickelous hydroxide. The blue ammoniacal solution of
nickel resembles the same solution of cupric oxide, but has a somewhat
reddish tint. It is characterised by the fact that it dissolves silk in
the same way as the ammoniacal cupric oxide dissolves cellulose. Ammonia
likewise dissolves the precipitate of cobaltous hydroxide, forming
a brownish liquid, which becomes darker in air and finally assumes a
bright red hue, absorbing oxygen. The admixture of ammonium chloride
prevents the precipitation of cobalt salts by ammonia; when the ammonia
is added, a brown solution is obtained from which, as in the
case of the preceding solution, potassium hydroxide does not separate
the cobaltous oxide. Peculiar compounds are produced in this solution;
they are comparatively stable, containing ammonia and an excess of
oxygen; they bear the name cobaltoamine and cobaltiamine salts. They
have been principally investigated by Genth, Frémy, Jörgenson and
others. Genth found that when a cobalt salt, mixed with an excess of
ammonium chloride, is treated with ammonia and exposed to the air,
after a certain lapse of time, on adding hydrochloric acid and boiling,
a red powder is precipitated and the remaining solution contains an
orange salt. The study of these compounds led to the discovery of a
whole series of similar salts, some of which correspond with particular
higher degrees of oxidation of cobalt, which are described later.[35][360]
[361]
[362]
[363]
[364]
[365]
Nickel does not possess this property of absorbing the oxygen of the air
when in an ammoniacal solution. In order to understand this distinction,
and in general the relation of nickel, it is important to observe
that cobalt more easily forms a higher degree of oxidation—namely,
sesquioxide of cobalt, cobaltic oxide, Co2O3—than nickel, especially in
the presence of hypochlorous acid. If a solution of a cobalt salt be
mixed with barium carbonate and an excess of hypochlorous acid be
added, or chlorine gas be passed through it, then at the ordinary
temperature on shaking, the whole of the cobalt will be separated
in the form of black cobaltic oxide: 2CoSO4 + ClHO + 2BaCO3
= Co2O3 + 2BaSO4 + HCl + 2CO2. Under these circumstances nickelous
oxide does not immediately form black sesquioxide, but after a considerable
space of time it also separates in the form of sesquioxide, Ni2O3,
but always later than cobalt. This is due to the relative difficulty of
further oxidation of the nickelous oxide. It is, however, possible to
oxidise it; if, for instance, the hydroxide NiH2O2 be shaken in water
and chlorine gas be passed through it, then nickel chloride will be
formed, which is soluble in water, and insoluble nickelic oxide in the
form of a black precipitate: 3NiH2O2 + Cl2 = NiCl2 + Ni2O3,3H2O.
Nickelic oxide may also be obtained by adding sodium hypochlorite
mixed with alkali to a solution of a nickel salt. Nickelic and cobaltic
hydrates are black. Nickelic oxide evolves oxygen with all acids, and
in consequence of this it is not separated as a precipitate in the presence
of acids; thus it evolves chlorine with hydrochloric acid, exactly like
manganese dioxide. When nickelic oxide is dissolved in aqueous
ammonia it liberates nitrogen, and an ammoniacal solution of nickelous
oxide is formed. When heated, nickelic oxide loses oxygen, forming[366]
nickelous oxide. Cobaltic oxide, Co2O3, exhibits more stability than
nickelic oxide, and shows feeble basic properties; thus it is dissolved
in acetic acid without the evolution of oxygen.[35 bis] But ordinary acids,
especially on heating, evolve oxygen, forming a solution of a cobaltous
salt. The presence of a cobaltic salt in a solution of a cobaltous salt
may be detected by the brown colour of the solution and the black
precipitate formed by the addition of alkali, and also from the fact that
such solutions evolve chlorine when heated with hydrochloric acid.
Cobaltic oxide may not only be prepared by the above-mentioned
methods, but also by heating cobalt nitrate, after which a steel-coloured
mass remains which retains traces of nitric acid, but when heated
further to incandescence evolves oxygen, leaving a compound of
cobaltic and cobaltous oxides, similar to magnetic ironstone. Cobalt
(but not nickel) undoubtedly forms besides Co2O3 a dioxide CoO2.
This is obtained[36] when the cobaltous oxide is oxidised by iodine or
peroxide of barium.[37]
[367]
Nickel alloys possess qualities which render them valuable for
technical purposes, the alloy of nickel with iron being particularly
remarkable. This alloy is met with in nature as meteoric iron. The
Pallasoffsky mass of meteoric iron, preserved in the St. Petersburg
Academy, fell in Siberia in the last century; it weighs about 15 cwt.
and contains 88 p.c. of iron and about 10 p.c. of nickel, with a
small admixture of other metals. In the arts German silver is most
extensively used; it is an alloy containing nickel, copper, and zinc in
various proportions. It generally consists of about 50 parts of copper,
25 parts of zinc, and 25 parts of nickel. This alloy is characterised by
its white colour resembling that of silver, and, like this latter metal, it
does not rust, and therefore furnishes an excellent substitute for silver
in the majority of cases where it is used. Alloys which contain silver
in addition to nickel show the properties of silver to a still greater
extent. Alloys of nickel are used for currency, and if rich deposits of
nickel are discovered a wide field of application lies before it, not only
in a pure state (because it is a beautiful metal and does not rust) but
also for use in alloys. Steel vessels (pressed or forged out of sheet
steel) covered with nickel have such practical merits that their manufacture,
which has not long commenced, will most probably be rapidly
developed, whilst nickel steel, which exceeds ordinary steel in its
tenacity, has already proved its excellent qualities for many purposes
(for instance, for armour plate).
Until 1890 no compound of cobalt or nickel was known of sufficient
volatility to determine the molecular weights of the compounds of these
metals; but in 1890 Mr. L. Mond, in conducting (together with Langer
and Quincke) his researches on the action of nickel upon carbonic oxide
(Chapter IX., Note 24 bis), observed that nickel gradually volatilises in
a stream of carbonic oxide; this only takes place at low temperatures,
and is seen by the coloration of the flame of the carbonic oxide. This
observation led to the discovery of a remarkable volatile compound of
nickel and carbonic oxide, having as molecular composition Ni(CO)4,[38][368]
as determined by the vapour density and depression of the freezing
point. Cobalt and many other metals do not form volatile compounds
under these conditions, but iron gives a similar product (Note 26 bis).
Ni(CO)4 is prepared by taking finely divided Ni (obtained by reducing
NiO by heating it in a stream of hydrogen, or by igniting the oxalate
NiC2O4)[39] and passing (at a temperature below 50°, for even at 60°
decomposition may take place and an explosion) a stream of CO over
it; the latter carries over the vapour of the compound, which condenses
(in a well-cooled receiver) into a perfectly colourless extremely mobile
liquid, boiling without decomposition at 43°, and crystallising in needles
at -25° (Mond and Nasini, 1891). Liquid Ni(CO)4 has a sp. gr. 1·356
at 0°, is insoluble in water, dissolves in alcohol and benzene, and burns
with a very smoky flame due to the liberation of Ni. The vapour when
passed through a tube heated to 180° and above deposits a brilliant
coating of metal, and disengages CO. If the tube be strongly heated
the decomposition is accompanied by an explosion. If Ni(CO)4 as
vapour be passed through a solution of CuCl2, it reduces the latter to
metal; it has the same action upon an ammoniacal solution of AgCl, strong
nitric acid oxidises Ni(CO)4, dilute solutions of acids have no action;
if the vapour be passed through strong sulphuric acid, CO is liberated,
chlorine gives NiCl and COCl2; no simple reactions of double decomposition
are yet known for Ni(CO)4, however, so that its connection
with other carbon compounds is not clear. Probably the formation of
this compound could be applied for extracting nickel from its ores.[40]
[369]
CHAPTER XXIII
THE PLATINUM METALS
The six metals: ruthenium, Ru, rhodium, Rh, palladium, Pd, osmium,
Os, iridium, Ir, and platinum, Pt, are met with associated together in
nature. Platinum always predominates over the others, and hence
they are known as the platinum metals. By their chemical character
their position in the periodic system is in the eighth group, corresponding
with iron, cobalt, and nickel.
The natural transition from titanium and vanadium to copper and
zinc by means of the elements of the iron group is demonstrated by all
the properties of these elements, and in exactly the same manner a
transition from zirconium, niobium, and molybdenum to silver, cadmium,
and indium, through ruthenium, rhodium, and palladium, is in perfect
accordance with fact and with the magnitude of the atomic weights, as
also is the position of osmium, iridium, and platinum between tantalum
and tungsten on the one side, and gold and mercury on the other. In
all these three cases the elements of smaller atomic weight (chromium,
molybdenum, and tungsten) are able, in their higher grades of
oxidation, to give acid oxides having the properties of distinct but
feebly energetic acids (in the lower oxides they give bases), whilst the
elements of greater atomic weight (zinc, cadmium, mercury), even in
their higher grades of oxidation, only give bases, although with feebly
developed basic properties. The platinum metals present the same
intermediate properties such as we have already seen in iron and the
elements of the eighth group.
In the platinum metals the intermediate properties of feebly acid
and feebly basic metals are developed with great clearness, so that
there is not one sharply-defined acid anhydride among their oxides,
although there is a great diversity in the grades of oxidation from the
type RO4 to R2O. The feebleness of the chemical forces observed in
the platinum metals is connected with the ready decomposability of
their compounds, with the small atomic volume of the metals themselves,[370]
and with their large atomic weight. The oxides of platinum,
iridium, and osmium can scarcely be termed either basic or acid; they
are capable of combinations of both kinds, each of which is feeble.
They are all intermediate oxides.
The atomic weights of platinum, iridium, and osmium are nearly
191 to 196, and of palladium, rhodium, and ruthenium, 104 to 106.
Thus, strictly speaking, we have here two series of metals, which
are, moreover, perfectly parallel to each other; three members in
the first series, and three members in the second—namely, platinum
presents an analogy to palladium, iridium to rhodium, and osmium
to ruthenium. As a matter of fact, however, the whole group of the
platinum metals is characterised by a number of common properties,
both physical and chemical, and, moreover, there are several points of
resemblance between the members of this group and those of the iron
group (Chapter XXII.) The atomic volumes (Table III., column 18)
of the elements of this group are nearly equal and very small. The iron
metals have atomic volumes of nearly 7, whilst that of the metals allied
to palladium is nearly 9, and of those adjacent to platinum (Pt, Ir, Os)
nearly 9·4. This comparatively small atomic volume corresponds with
the great infusibility and tenacity proper to all the iron and platinum
metals, and to their small chemical energy, which stands out very
clearly in the heavy platinum metals. All the platinum metals are
very easily reduced by ignition and by the action of various reducing
agents, in which process oxygen, or a haloid group, is disengaged from
their compounds and the metal left behind. This is a property of the
platinum metals which determines many of their reactions, and the
circumstance of their always being found in nature in a native state.
In Russia in the Urals (discovered in 1819) and in Brazil (1735)
platinum is obtained from alluvial deposits, but in 1892 Professor
Inostrantseff discovered a vein deposit of platinum in serpentine near
Tagil in the Urals.[1] The facility with which they are reduced is so
great that their chlorides are even decomposed by gaseous hydrogen,
especially when shaken up and heated under a certain pressure. Hence
it will be readily understood that such metals as zinc, iron, &c., separate
them from solutions with great ease, which fact is taken advantage of
in practice and in the chemical treatment of the platinum metals.[1 bis]
[371]
All the platinum metals, like those of the iron group, are grey, with
a comparatively feeble metallic lustre, and are very infusible. In this
respect they stand in the same order as the metals of the iron series;
nickel is more fusible and whiter than cobalt and iron, so also palladium
is whiter and more fusible than rhodium and ruthenium, and
platinum is comparatively more fusible and whiter than iridium or
osmium. The saline compounds of these metals are red or yellow, like
those of the majority of the metals of the iron series, and like the
latter, the different forms of oxidation present different colours. Moreover,
certain complex compounds of the platinum metals, like certain
complex compounds of the iron series, either have particular characteristic
tints or else are colourless.
The platinum metals are found in nature associated together in
alluvial deposits in a few localities, from which they are washed,
owing to their very considerable density, which enables a stream of
water to wash away the sand and clay with which they are mixed.
Platinum deposits are chiefly known in the Urals, and also in Brazil
and a few other localities. The platinum ore washed from these
alluvial deposits presents the appearance of more or less coarse grains,
and sometimes, as it were, of semi-fused nuggets.[2]
All the platinum metals give compounds with the halogens, and the
highest haloid type of combination for all is RX4. For the majority
of the platinum metals this type is exceedingly unstable; the lower
compounds corresponding to the type RX2, which are formed by the
separation of X2, are more stable. In the type RX2 the platinum
metals form more stable salts, which offer no little resemblance to[372]
the kindred compounds of the iron series—for example, to nickelous
chloride, NiCl2, cobaltous chloride, CoCl2, &c. This even expresses
itself in a similarity of volume (platinous chloride, PtCl2, volume, 46;
nickelous chloride, NiCl2 = 50), although in the type RX2 the true iron
metals give very stable compounds, whilst the platinum metals frequently
react after the manner of suboxides, decomposing into the
metal and higher types, 2RX2 = R + RX4. This probably depends on
the facility with which RX2 decomposes into R and X2, when X2
combines with the remaining portion of RX2.
As in the series iron, cobalt, nickel, nickel gives NiO and Ni2O3,
whilst cobalt and iron give higher and varied forms of oxidation, so
also among the platinum metals, platinum and palladium only give the
forms RX2 and RX4, whilst rhodium and iridium form another and
intermediate type, RX3, also met with in cobalt, corresponding with
the oxide, having the composition R2O3, besides which they form
an acid oxide, like ferric acid, which is also known in the form of
salts, but is in every respect unstable. Osmium and ruthenium, like
manganese, form still higher oxides, and in this respect exhibit the
greatest diversity. They not only give RX2, RX3, RX4, and RX6,
but also a still higher form of oxidation, RO4, which is not met with in
any other series. This form is exceedingly characteristic, owing to the
fact that the oxides, OsO4 and RuO4, are volatile and have feebly acid
properties. In this respect they most resemble permanganic anhydride,
which is also somewhat volatile.[3]
When dissolved in aqua regia (PtCl4 is formed) and liberated from
the solution by sal-ammoniac ((NH4)2PtCl6 is formed) and reduced by
ignition (which may be done by Zn and other reducing agents, direct
from a solution of PtCl4) platinum[3 bis] forms a powdery mass, known[373]
as spongy platinum or platinum black. If this powder of platinum be
heated and pressed, or hammered in a cylinder, the grains aggregate or
forge together, and form a continuous, though of course not entirely
homogeneous, mass. Platinum was formerly, and is even now, worked
up in this manner. The platinum money formerly used in Russia was
made in this way. Sainte-Claire Deville, in the fifties, for the first
time melted platinum in considerable quantities by employing a special
furnace made in the form of a small reverberatory furnace, and composed
of two pieces of lime, on which the heat of the oxyhydrogen flame
has no action. Into this furnace (shown in fig. 34, Vol. I. p. 175)—or,
more strictly speaking, into the cavity made in the pieces of lime—the
platinum is introduced, and two orifices are made in the lime; through
one, the upper, or side orifice, is introduced an oxyhydrogen gas burner,
in which either detonating gas or a mixture of oxygen and coal-gas is
burnt, whilst the other orifice serves for the escape of the products of
combustion and certain impurities which are more volatile than the
platinum, and especially the oxidised compounds of osmium, ruthenium,
and palladium, which are comparatively easily volatilised by heat. In
this manner the platinum is converted into a continuous metallic form
by means of fusion, and this method is now used for melting considerable
masses of platinum[4] and its alloys with iridium.
[374]
To obtain pure platinum, the ore is treated with aqua regia in which
only the osmium and iridium are insoluble. The solution contains the
platinum metals in the form RCl4, and in the lower forms of chlorination,
RCl3 and RCl2, because some of these metals—for instance,
palladium and rhodium—form such unstable chlorides of the type RX4
that they partially decompose even when diluted with water, and pass
into the stable lower type of combination; in addition to which the
chlorine is very easily disengaged if it comes in contact with substances
on which it can act. In this respect platinum resists the action of
heat and reducing agents better than any of its companions—that is,
it passes with greater difficulty from PtCl4 to the lower compound
PtCl2. On this is based the method of preparation of more or less
pure platinum. Lime or sodium hydroxide is added to the solution in
aqua regia until neutralised, or only containing a very slight excess of
alkali. It is best to first evaporate and slightly ignite the solution, in
order to remove the excess of acid, and by heating it to partially convert
the higher chlorides of the palladium, &c., into the lower. The
addition of alkalis completes the reduction, because the chlorine held
in the compounds RX4 acts on the alkali like free chlorine, converting
it into a hypochlorite. Thus palladium chloride, PdCl4, for example,
is converted into palladious chloride, PdCl2, by this means, according
to the equation PdCl4 + 2NaHO = PdCl2 + NaCl + NaClO + H2O. In
a similar manner iridic chloride, IrCl4, is converted into the trichloride,
IrCl3, by this method. When this conversion takes place the platinum
still remains in the form of platinic chloride, PtCl4. It is then possible
to take advantage of a certain difference in the properties of the higher
and lower chlorides of the platinum metals. Thus lime precipitates the
lower chlorides of the members of the platinum metals occurring in
solution without acting on the platinic chloride, PtCl4, and hence the
addition of a large proportion of lime immediately precipitates the
associated metals, leaving the platinum itself in solution in the form
of a soluble double salt, PtCl4,CaCl2. A far better and more perfect[375]
separation is effected by means of ammonium chloride, which gives, with
platinic chloride, an insoluble yellow precipitate, PtCl4,2NH4Cl, whilst
it forms soluble double salts with the lower chlorides RCl2 and RCl3,
so that ammonium chloride precipitates the platinum only from the
solution obtained by the preceding method. These methods are
employed for preparing the platinum which is used for the manufacture
of platinum articles, because, having platinum in solution as calcium
platinochloride, PtCaCl6, or as the insoluble ammonium platinochloride,
Pt(NH4)2Cl6, the platinum compound in every case, after drying or
ignition, loses all the chlorine from the platinic chloride and leaves finely-divided
metallic platinum, which may be converted into homogeneous
metal by compression and forging, or by fusion.[5]
[376]
Metallic platinum in a fused state has a specific gravity of 21; it
is grey, softer than iron but harder than copper, exceedingly ductile,
and therefore easily drawn into wire and rolled into thin sheets, and
may be hammered into crucibles and drawn into thin tubes, &c. In
the state in which it is obtained by the ignition of its compounds,
it forms a spongy mass, known as spongy platinum, or else as powder
(platinum black).[6] In either case it is dull grey, and is characterised,
as we already know, by the faculty of absorbing hydrogen and other
gases. Platinum is not acted on by hydrochloric, hydriodic, nitric, and
sulphuric acids, or a mixture of hydrofluoric and nitric acids. Aqua
regia, and any liquid containing chlorine or able to evolve chlorine or
bromine, dissolves platinum. Alkalis are decomposed by platinum at
a red heat, owing to the faculty of the platinum oxide, PtO2, formed to
combine with alkaline bases, inasmuch as it has a feebly-developed acid
character (see Note 8). Sulphur, phosphorus (the phosphide, PtP2,[377]
is formed), arsenic and silicon all act more or less rapidly on platinum,
under the influence of heat. Many of the metals form alloys with it.
Even charcoal combines with platinum when it is ignited with it, and
therefore carbonaceous matter cannot be subjected to prolonged and
powerful ignition in platinum vessels. Hence a platinum crucible soon
becomes dull on the surface in a smoky flame. Platinum also forms
alloys with zinc, lead, tin, copper, gold, and silver.[7] Although mercury
does not directly dissolve platinum, still it forms a solution or amalgam
with spongy platinum in the presence of sodium amalgam; a similar
amalgam is also formed by the action of sodium amalgam on a solution
of platinum chloride, and is used for physical experiments.
There are two kinds of platinum compounds, PtX4 and PtX2.
The former are produced by an excess of halogen in the cold, and the
latter by the aid of heat or by the splitting up of the former. The
starting-point for the platinum compounds is platinum tetrachloride,
platinic chloride, PtCl4, obtained by dissolving platinum in aqua
regia.[7 bis] The solution crystallises in the cold, in a desiccator, in the
form of reddish-brown deliquescent crystals which contain hydrochloric
acid, PtCl4,2HCl,6H2O, and behave like a true acid whose salts correspond
to the formula R2PtCl6—ammonium platinochloride, for
example.[7 tri] The hydrochloric acid is liberated from these crystals by
gently heating or evaporating the solution to dryness; or, better still,
after treatment with silver nitrate a reddish-brown mass remains
behind, which dissolves in water, and forms a yellowish-red solution
which on cooling deposits crystals of the composition PtCl4,8H2O.
The tendency of PtCl4 to combine with hydrochloric acid and water—that
is, to form higher crystalline compounds—is evident in the
platinum compounds, and must be taken into account in explaining
the properties of platinum and the formation of many other of its
complex compounds. Dilute solutions of platinic chloride are yellow,
and are completely reduced by hydrogen, sulphurous anhydride, and
many reducing agents, which first convert the platinic chloride into[378]
the lower compound platinous chloride, PtCl2. That faculty which
reveals itself in platinum tetrachloride of combining with water of
crystallisation and hydrochloric acid is distinctly marked in its property,
with which we are already acquainted, of giving precipitates
with the salts of potassium, ammonium, rubidium, &c. In general it
readily forms double salts, R2PtCl6 = PtCl4 + 2RCl, where R is a
univalent metal such as potassium or NH4. Hence the addition of a
solution of potassium or ammonium chloride to a solution of platinic
chloride is followed by the formation of a yellow precipitate, which is
sparingly soluble in water and almost entirely insoluble in alcohol and
ether (platinic chloride is soluble in alcohol, potassium iridiochloride,
IrK3Cl6, i.e. a compound of IrCl3, is soluble in water but not in alcohol).
It is especially remarkable in this case, that the potassium compounds
here, as in a number of other instances, separate in an anhydrous form,
whilst the sodium compounds, which are soluble in water and alcohol,
form red crystals containing water. The composition Na2PtCl6,6H2O
exactly corresponds with the above-mentioned hydrochloric compound.
The compounds with barium, BaPtCl6,4H2O, strontium, SrPtCl6,8H2O,
calcium, magnesium, iron, manganese, and many other metals are all
soluble in water.[8]
[379]
Platinous chloride, PtCl2, is formed when hydrogen platinochloride,
PtH2Cl6, is ignited at 300°, or when potassium is heated at 230° in a
stream of chlorine. The undecomposed tetrachloride is extracted from
the residue by washing it with water, and a greenish-grey or brown
insoluble mass of the dichloride (sp. gr. 5·9) is then obtained. It is
soluble in hydrochloric acid, giving an acid solution of the composition
PtCl2,2HCl, corresponding with the type of double salts PtR2Cl4.
Although platinous chloride decomposes below 500°, still it is formed to
a small extent at higher temperatures. Troost and Hautefeuille, and
Seelheim observed that when platinum was strongly ignited in a stream of
chlorine, the metal, as it were, slowly volatilised and was deposited in
crystals; a volatile chloride, probably platinous chloride, was evidently
formed in this case, and decomposed subsequently to its formation,
depositing crystals of platinum.
The properties of platinum above-described are repeated more or less
distinctly, or sometimes with certain modifications, in the above-mentioned
associates and analogues of this metal. Thus although palladium
forms PdCl4, this form passes into PdCl2 with extreme ease.[9] Whilst[380]
[381]
[382]
rhodium and iridium in dissolving in aqua regia also form RhCl4 and
IrCl4, but they pass into RhCl3 and IrCl3[9 bis] very easily when heated
or when acted upon by substances capable of taking up chlorine (even
alkalis, which form bleaching salts). Among the platinum metals,
ruthenium and osmium have the most acid character, and although they
give RuCl4 and OsCl4 they are easily oxidised to RuO4, and OsO4 by
the action of chlorine in the presence of water; the latter are volatile
and may be distilled with the water and hydrochloric acid, from a
solution containing other platinum metals.[9 tri] Thus with respect to[383]
the types of combination, all the platinum metals, under certain circumstances,
give compounds of the type RX4—for instance, RO2, RCl4, &c.[384]
But this is the highest form for only platinum and palladium.
The remaining platinum metals further, like iron, give acids of the type[385]
RO3 or hydrates, H2RO4 = RO2(HO)2 (the type of sulphuric acid); but
they, like ferric and manganic acids, are chiefly known in the form of
salts of the composition K2RO4 or K2R2O7 (like the dichromate). These
salts are obtained, like the manganates and ferrates, by fusing the oxides,
or even the metals themselves, with nitric, or, better still, with potassium
peroxide. They are soluble in water, are easily deoxidised and do not
yield the acid anhydrides under the action of acids, but break up, either
(like the ferrate) forming oxygen and a basic oxide (iridium and rhodium
react in this manner, as they do not give higher forms of oxidation), or
passing into a lower and higher form of oxidation—that is, reacting
like a manganate (or partly like nitrite or phosphite). Osmium and
ruthenium react according to the latter form, as they are capable of
giving higher forms of oxidation, OsO4 and RuO4, and therefore their
reactions of decomposition may be essentially represented by the equation:
2OsO3 = OsO2 + OsO4.[10]
[386]
Platinum and its analogues, like iron and its analogues, are able to
form complex and comparatively stable cyanogen and ammonia compounds,
corresponding with the ferrocyanides and the ammoniacal compounds
of cobalt, which we have already considered in the preceding
chapter.
If platinous chloride, PtCl2 (insoluble in water), be added by degrees
to a solution of potassium cyanide, it is completely dissolved (like
silver chloride), and on evaporating the solution deposits rhombic
prisms of potassium platinocyanide, PtK2(CN)4,3H2O. This salt, like
all those corresponding with it, has a remarkable play of colours, due to
the phenomena of dichromism, and even polychromism, natural to all
the platinocyanides. Thus it is yellow and reflects a bright blue
light. It is easily soluble in water, effloresces in air, then turns red,
and at 100° orange, when it loses all its water. The loss of water
does not destroy its stability—that is, it still remains unchanged, and
its stability is further shown by the fact that it is formed when
potassium ferrocyanide, K4Fe(CN)6, is heated with platinum black.
This salt, first obtained by Gmelin, shows a neutral reaction with
litmus; it is exceedingly stable under the action of air, like potassium
ferrocyanide, which it resembles in many respects. Thus the platinum
in it cannot be detected by reagents such as sulphuretted hydrogen;
the potassium may be replaced by other metals by the action of their
salts, so that it corresponds with a whole series of compounds, R2Pt(CN)4,
and it is stable, although the potassium cyanide and platinous salts, of
which it is composed, individually easily undergo change. When
treated with oxidising agents it passes, like the ferrocyanide, into a
higher form of combination of platinum. If salts of silver be added
to its solution, it gives a heavy white precipitate of silver platinocyanide,
PtAg2(CN)4, which when suspended in water and treated
with sulphuretted hydrogen, enters into double decomposition with the
latter and forms insoluble silver sulphide, Ag2S, and soluble hydroplatinocyanic
acid, H2Pt(CN)4. If potassium platinocyanide be mixed
with an equivalent quantity of sulphuric acid, the hydroplatinocyanic
acid liberated may be extracted by a mixture of alcohol and
ether. The ethereal solution, when evaporated in a desiccator, deposits
bright red crystals of the composition PtH2(CN)4,5H2O. This acid
colours litmus paper, liberates carbonic anhydride from sodium carbonate,
and saturates alkalis, so that it presents an analogy to hydroferrocyanic
acid.[11]
[387]
[388]
[389]
[390]
[391]
Ammonia, like potassium cyanide, has the faculty of easily reacting
with platinum dichloride, forming compounds similar to the platinocyanide
and cobaltia compounds, which are comparatively stable. But
as ammonia does not contain any hydrogen easily replaceable by
metals, and as ammonia itself is able to combine with acids, the
PtX2 plays, as it were, the part of an acid with reference to the
ammonia. Owing to the influence of the ammonia, the X2 in the
resultant compound will represent the same character as it has in
ammoniacal salts; consequently, the ammoniacal compounds produced
from PtX2 will be salts in which X will be replaceable by various
other haloids, just as the metal is replaced in the cyanogen salts; such
is the nature of the platino-ammonium compounds. PtX2 forms compounds
with 2NH3 and with 4NH3, and so also PtX4 gives (not
directly from PtX4 and ammonia, but from the compounds of PtX2 by
the action of chlorine, &c.) similar compounds with 2NH3 and with
4NH3.[12]
[392]
[393]
If ammonia acts on a boiling solution of platinous chloride in
hydrochloric acid, it produces the green salt of Magnus (1829),
PtCl2,2NH3, insoluble in water and hydrochloric acid. But, judging
by its reactions, this salt has twice this formula. Thus, Gros (1837),
on boiling Magnus's salt with nitric acid, observed that half the chlorine
was replaced by the residue of nitric acid and half the platinum was
disengaged: 2PtCl2(NH3)2 + 2HNO3 = PtCl2(NO3)2(NH3)4 + 2PtCl2.
The Gros's salt thus obtained, PtCl2(NO3)24NH3 (if Magnus's salt[394]
belongs to the type PtX2, then Gros's salt belongs to the type PtX4), is
soluble in water, and the elements of nitric acid, but not the chlorine,
contained in it are capable of easily submitting themselves to double
saline decomposition. Thus silver nitrate does not enter into double
decomposition with the chlorine of Gros's salt. Most instructive was
the circumstance that Gros, by acting on his salt with hydrochloric
acid, succeeded in substituting the residue of nitric acid in it by
chlorine, and the chlorine thus introduced, easily reacted with silver
nitrate. Thus it appeared that Gros's salt contained two varieties of
chlorine—one which reacts readily, and the other which reacts with
difficulty. The composition of Gros's first salt is PtCl2(NH3)4(NO3)2;
it may be converted into PtCl2(NH3)4(SO4), and in general into
PtCl2(NH3)4X2.[13]
The salt of Magnus when boiled with a solution of ammonia gives
the salt (of Reiset's first base) PtCl2(NH3)4, and this, when treated
with bromine, forms the salt PtCl2Br2(NH3)4, which has the same
composition and reactions as Gros's salt. To Reiset's salts there
corresponds a soluble, colourless, crystalline hydroxide, Pt(OH)2(NH3)4,
having the properties of a powerful and very energetic alkali; it
attracts carbonic anhydride from the atmosphere, precipitates metallic
salts like potash, saturates active acids, even sulphuric, forming
colourless (with nitric, carbonic, and hydrochloric acids), or yellow
(with sulphuric acid), salts of the type PtX2(NH3)4.[14] The comparative[395]
stability (for instance, as compared with AgCl and NH3) of
such compounds, and the existence of many other compounds analogous[396]
[397]
to them, endows them with a particular chemical interest. Thus
Kournakoff (1889) obtained a series of corresponding compounds containing
thiocarbamide, CSN2H4, in the place of ammonia, PtCl2,4CSN2H4,
and others corresponding with Reiset's salts. Hydroxylamine, and
other substances corresponding with ammonia, also give similar compounds.
The common properties and composition of such compounds
show their entire analogy to the cobaltia compounds (especially for
ruthenium and iridium) and correspond to the fact that both the
platinum metals and cobalt occur in the same, eighth, group.
[398]
CHAPTER XXIV
COPPER, SILVER, AND GOLD
That degree of analogy and difference which exists between iron,
cobalt, and nickel repeats itself in the corresponding triad ruthenium,
rhodium, and palladium, and also in the heavy platinum metals,
osmium, iridium, and platinum. These nine metals form Group VIII.
of the elements in the periodic system, being the intermediate group
between the even elements of the large periods and the uneven, among
which we know zinc, cadmium, and mercury in Group II. Copper,
silver, and gold complete[1] this transition, because their properties
place them in proximity to nickel, palladium, and platinum on the one
hand, and to zinc, cadmium, and mercury on the other. Just as Zn,
Cd, and Hg; Fe, Ru, and Os; Co, Rh, and Ir; Ni, Pd, and Pt,
resemble each other in many respects, so also do Cu, Ag, and Au.
Thus, for example, the atomic weight of copper Cu = 63, and in all its
properties it stands between Ni = 59 and Zn = 65. But as the transition
from Group VIII. to Group II., where zinc is situated, cannot be
otherwise than through Group I., so in copper there are certain properties
of the elements of Group I. Thus it gives a suboxide, Cu2O,
and salts, CuX, like the elements of Group I., although at the same
time it forms an oxide, CuO, and salts CuX2, like nickel and zinc. In
the state of the oxide, CuO, and the salts, CuX2, copper is analogous to
zinc, judging from the insolubility of the carbonates, phosphates, and
similar salts, and by the isomorphism, and other characters.[2] In the
cuprous salts there is undoubtedly a great resemblance to the silver[399]
salts—thus, for example, silver chloride, AgCl, is characterised by its
insolubility and capacity of combining with ammonia, and in this respect
cuprous chloride closely resembles it, for it is also insoluble in water,
and combines with ammonia and dissolves in it, &c. Its composition is
also RCl, the same as AgCl, NaCl, KCl, &c., and silver in many compounds
resembles, and is even isomorphous with, sodium, so that this
again justifies their being brought together. Silver chloride, cuprous
chloride, and sodium chloride crystallise in the regular system.
Besides which, the specific heats of copper and silver require that they
should have the atomic weights ascribed to them. To the oxides Cu2O
and Ag2O there are corresponding sulphides Ag2S and Cu2S. They
both occur in nature in crystals of the rhombic system, and, what is
most important, copper glance contains an isomorphous mixture of
them both, and retains the form of copper glance with various proportions
of copper and silver, and therefore has the composition R2S
where R = Cu, Ag.
Notwithstanding the resemblance in the atomic composition of the
cuprous compounds, CuX, and silver compounds, AgX, with the compounds
of the alkali metals KX, NaX, there is a considerable degree
of difference between these two series of elements. This difference is
clearly seen in the fact that the alkali metals belong to those elements
which combine with extreme facility with oxygen, decompose water,
and form the most alkaline bases; whilst silver and copper are
oxidised with difficulty, form less energetic oxides, and do not decompose
water, even at a rather high temperature. Moreover, they only
displace hydrogen from very few acids. The difference between them
is also seen in the dissimilarity of the properties of many of the
corresponding compounds. Thus cuprous oxide, Cu2O, and silver oxide,
Ag2O, are insoluble in water: the cuprous and silver carbonates,
chlorides, and sulphates are also sparingly soluble in water. The
oxides of silver and copper are also easily reduced to metal. This
difference in properties is in intimate relation with that difference in
the density of the metals which exists in this case. The alkali metals
belong to the lightest, and copper and silver to the heaviest, and therefore
the distance between the molecules in these metals is very dissimilar—it
is greater for the former than the latter (tables in Chapter
XV.). From the point of view of the periodic law, this difference
between copper and silver and such elements of Group I. as potassium
and rubidium, is clearly seen from the fact that copper and silver[400]
stand in the middle of those large periods (for example, K, Ca, Sc, Ti,
V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br) which start with
the true metals of the alkalis—that is to say, the analogy and difference
between potassium and copper are of the same nature as that between
chromium and selenium, or vanadium and arsenic.
Copper is one of the few metals which have long been known in a
metallic form. The Greeks and Romans imported copper chiefly from
the island of Cyprus—whence its Latin name, cuprum. It was known
to the ancients before iron, and was used, especially when alloyed with
other metals, for arms and domestic utensils. That copper was known
to the ancients will be understood from the fact that it occurs, although
rarely, in a native state, and is easily extracted from its other natural
compounds. Among the latter are the oxygen compounds of copper.
When ignited with charcoal, they easily give up their oxygen to
it, and yield metallic copper; hydrogen also easily takes up the
oxygen from copper oxide when heated. Copper occurs in a native
state, sometimes in association with other ores, in many parts of the
Urals and in Sweden, and in considerable masses in America, especially
in the neighbourhood of the great American lakes; and also in
Chili, Japan, and China. The oxygen compounds of copper are also of
somewhat common occurrence in certain localities; in this respect
certain deposits of the Urals are especially famous. The geological
period of the Urals (Permian) is characterised by a considerable distribution
of copper ores. Copper is met with in the form of cuprous
oxide, or suboxide of copper, Cu2O, and is then known as red copper
ore, because it forms red masses which not unfrequently are crystallised
in the regular system. It is found much more rarely in the state of
cupric oxide, CuO, and is then called black copper ore. The most
common of the oxygenised compounds of copper are the basic carbonates
corresponding with the oxides. That these compounds are undoubtedly
of aqueous origin is apparent, not only from the fact that specimens
are frequently found of a gradual transition from the metallic, sulphuretted,
and oxidised copper into its various carbonates, but also from
the presence of water in their composition, and from the laminar,
reniform structure which many of them present. In this respect malachite
is particularly well known; it is used as a green paint and also
for ornaments, owing to the diversity of the shades of colour presented
by the different layers of deposited malachite. The composition of
malachite corresponds with the basic carbonate containing one molecule
of cupric carbonate to one of hydroxide: CuCO3,CuH2O2. In this
form the copper frequently occurs in admixture with various sedimentary
rocks, forming large strata, which confirms the aqueous origin[401]
of these compounds. There are many such localities in the Perm and
other Governments bounding the Urals. Blue carbonate of copper, or
azurite, is also often met with in the same localities; it contains the
same ingredients as malachite, but in a different proportion, its composition
being CuH2O2,2CuCO3. Both these substances may be obtained
artificially by the action of the alkali carbonates on solutions
of cupric salts at various temperatures. These native carbonates are
often used for the extraction of copper, all the more as they very
readily give metallic copper, evolving water and carbonic anhydride
when ignited, and leaving the easily-reducible cupric oxide. Copper
is, however, still more often met with in the form of the sulphides.
The sulphides of copper generally occur in chemical combination
with the sulphides of iron.[3] These copper-sulphur compounds (copper
pyrites CuFeS2, variegated copper ore Cu3FeS3, &c.) generally occur in
veins in a rock gangue.
The extraction of copper from its oxide ores does not present any
difficulty, because the copper, when ignited with charcoal and melted,
is reduced from the impurities which accompany it. This mode of
smelting copper ores is carried on in cupola or cylindrical furnaces,
fluxes forming a slag being added to the mixture of ore and charcoal.[402]
The smelted copper still contains sulphur, iron, and other metallic
impurities, from which it is freed by fusion in reverberatory furnaces,
with access of air to the surface of the molten metal, as the iron and
sulphur are more easily oxidised than the copper. The iron then
separates as oxides, which collect in the slag.[4]
[403]
Copper is characterised by its red colour, which distinguishes it
from all other metals. Pure copper is soft, and may be beaten out by
a hammer at the ordinary temperature, and when hot may be rolled
into very thin sheets. Extremely thin leaves of copper transmit a
green light. The tenacity of copper is also considerable, and next to
iron it is one of the most durable metals in this respect. Copper wire
of 1 sq. millimetre in section only breaks under a weight of 45 kilograms.
The specific gravity of copper is 8·8, unless it contains cavities due to the
fact that molten copper absorbs oxygen from the air, which is disengaged
on cooling, and therefore gives a porous mass whose density is
much less. Rolled copper, and also that which is deposited by the electric
current, has a comparatively high density. Copper melts at a bright
red heat, about 1050°, although below the temperature at which many
kinds of cast iron melt. At a high temperature it is converted into
vapour, which communicates a green colour to the flame. Both native
copper and that cooled from a molten state crystallise in regular
octahedra. Copper is not oxidised in dry air at the ordinary temperature,
but when calcined it becomes coated with a layer of oxide, and it
does not burn even at the highest temperature. Copper, when calcined
in air, forms either the red cuprous oxide or the black cupric oxide,[404]
according to the temperature and quantity of air supplied. In air
at the ordinary temperature, copper—as everyone knows—becomes
coated with a brown layer of oxides or a green coating of basic salts,
due to the action of the damp air containing carbonic acid. If this
action continue for a prolonged time, the copper is covered with a thick
coating of basic carbonate, or the so-called verdigris (the ærugo nobilis
of ancient statues). This is due to the fact that copper, although
scarcely capable of oxidising by itself,[5] in the presence of water and
acids—even very feeble acids, like carbonic acid—absorbs oxygen from
the air and forms salts, which is a very characteristic property of it (and
of lead).[6] Copper does not decompose water, and therefore does not disengage[405]
hydrogen from it either at the ordinary or at high temperatures.
Nor does copper liberate hydrogen from the oxygen acids; these act on
it in two ways: they either give up a portion of their oxygen, forming
lower grades of oxidation, or else only react in the presence of
air. Thus, when nitric acid acts on copper it evolves nitric oxide, the
copper being oxidised at the expense of the nitric acid. In the same
way copper converts sulphuric acid into the lower grade of oxidation—into
sulphurous anhydride, SO2. In these cases the copper is oxidised
to copper oxide, which combines with the excess of acid taken, and
therefore forms a cupric salt, CuX2. Dilute nitric acid does not act
on copper at the ordinary temperature, but when heated it reacts
with great ease; dilute sulphuric acid does not act on copper except
in presence of air.
Both the oxides of copper, Cu2O and CuO, are unacted on by
air, and, as already mentioned, they both occur in nature.[6 bis] However,
in the majority of cases copper is obtained in the form of
cupric oxide and its salts—and the copper compounds used industrially
generally belong to this type. This is due to the fact that the
cuprous compounds absorb oxygen from the air and pass into cupric
compounds. The cupric compounds may serve as the source for the
preparation of cuprous oxide, because many reducing agents are
capable of deoxidising the oxide into the suboxide. Organic substances
are most generally employed for this purpose, and especially
saccharine substances, which are able, in the presence of alkalis, to
undergo oxidation at the expense of the oxygen of the cupric oxide,
and to give acids which combine with the alkali: 2CuO - O = Cu2O.
In this case the deoxidation of the copper may be carried further and
metallic copper obtained, if only the reaction be aided by heat. Thus,
for example, a fine powder of metallic copper may be obtained by heating
an ammoniacal solution of cupric oxide with caustic potash
and grape sugar. But if the reducing action of the saccharine
substance proceed in the presence of a sufficient quantity of alkali in[406]
solution, and at not too high a temperature, cuprous oxide is obtained.
To see this reaction clearly, it is not sufficient to take any
cupric salt, because the alkali necessary for the reaction might precipitate
cupric oxide—it is necessary to add previously some substance
which will prevent this precipitation. Among such substances,
tartaric acid, C4H6O6, is one of the best. In the presence of a sufficient
quantity of tartaric acid, any amount of alkali may be added to a
solution of cupric salt without producing a precipitate, because a soluble
double salt of cupric oxide and alkali is then formed. If glucose (for
instance, honey or molasses) be added to such an alkaline tartaric
solution, and the temperature be slightly raised, it first gives a yellow
precipitate (this is cuprous hydroxide, CuHO), and then, on boiling,
a red precipitate of (anhydrous) cuprous oxide. If such a mixture
be left for a long time at the ordinary temperature, it deposits well-formed
crystals of anhydrous cuprous oxide belonging to the regular
system.[7]
[407]
Cupric chloride, CuCl2, when ignited, gives cuprous chloride, CuCl—i.e.
the salt corresponding with suboxide of copper—and therefore
cuprous chloride is always formed when copper enters into reaction
with chlorine at a high temperature. Thus, for example, when copper
is calcined with mercuric chloride, it forms cuprous chloride and vapours
of mercury. The same substance is obtained on heating metallic
copper in hydrochloric acid, hydrogen being disengaged; but this reaction
only proceeds with finely-divided copper, as hydrochloric acid acts
very feebly on compact masses of copper, and, in the presence of air,
gives cupric chloride. The green solution of cupric chloride is decolorised
by metallic copper, cuprous chloride being formed; but this
reaction is only accomplished with ease when the solution is very concentrated
and in the presence of an excess of hydrochloric acid to
dissolve the cuprous chloride. The addition of water to the solution
precipitates the cuprous chloride, because it is less soluble in
dilute than in strong hydrochloric acid. Many reducing agents which
are able to take up half the oxygen from cupric oxide are able, in the
presence of hydrochloric acid, to form cuprous chloride. Stannous
salts, sulphurous anhydride, alkali sulphites, phosphorous and hypophosphorous
acids, and many similar reducing agents, act in this
manner. The usual method of preparing cuprous chloride consists in
passing sulphurous anhydride into a very strong solution of cupric
chloride: 2CuCl2 + SO2 + 2H2O = 2CuCl + 2HCl + H2SO4. Cuprous
chloride forms colourless cubic crystals which are insoluble in water.
It is easily fusible, and even volatile. Under the action of oxidising
agents, it passes into the cupric salt, and it absorbs oxygen from moist
air, forming cupric oxychloride, Cu2Cl2O. Aqueous ammonia easily
dissolves cuprous chloride as well as cuprous oxide; the solution also
turns blue on exposure to the air. Thus an ammoniacal solution of
cuprous chloride serves as an excellent absorbent for oxygen; but this
solution absorbs not only oxygen, but also certain other gases—for
example, carbonic oxide and acetylene.[8]
[408]
When copper is oxidised with a considerable quantity of oxygen at
a high temperature, or at the ordinary temperature in the presence of
acids, and also when it decomposes acids, converting them into lower
grades of oxidation (for example, when submitted to the action of
nitric and sulphuric acids), it forms cupric oxide, CuO, or, in the
presence of acids, cupric salts. Copper rust, or that black mass which
forms on the surface of copper when it is calcined, consists of cupric
oxide. The coating of the oxidised copper is very easily separated
from the metallic copper, because it is brittle and very easily peels off,
when it is struck or immersed in water. Many copper salts (for[409]
instance, the nitrite and carbonate) leave oxide of copper[8 bis] in the
form of friable black powder, after being ignited. If the ignition be
carried further, Cu2O may be formed from the CuO.[8 tri] Anhydrous
cupric oxide is very easily dissolved in acids, forming cupric salts, CuX2.
They are analogous to the salts MgX2, ZnX2, NiX2, FeX2, in many
respects. On adding potassium or ammonium hydroxide to a solution
of a cupric salt, it forms a gelatinous blue precipitate of the hydrated
oxide of copper, CuH2O2, insoluble in water. The resultant precipitate
is re-dissolved by an excess of ammonia, and gives a very beautiful
azure blue solution, of so intense a colour that the presence of small
traces of cupric salts may be discovered by this means.[9] An excess of[410]
potassium or sodium hydroxide does not dissolve cupric hydroxide.
A hot solution gives a black precipitate of the anhydrous oxide
instead of the blue precipitate, and the precipitate of the hydroxide
of copper becomes granular, and turns black when the solution
is heated. This is due to the fact that the blue hydroxide is
exceedingly unstable, and when slightly heated it loses the elements
of water and gives the black anhydrous cupric oxide: CuH2O2
= CuO + H2O.
Cupric oxide fuses at a strong heat, and on cooling forms a heavy
crystalline mass, which is black, opaque, and somewhat tenacious. It
is a feebly energetic base, so that not only do the oxides of the metals
of the alkalis and alkaline earths displace it from its compounds, but
even such oxides as those of lead and silver precipitate it from solutions,
which is partially due to these oxides being soluble, although but slightly
so, in water. However, cupric oxide, and especially the hydroxide,
easily combines with even the least energetic acids, and does not give
any compounds with bases; but, on the other hand, it easily forms
basic salts,[9 bis] and in this respect outstrips magnesium and recalls the[411]
oxides of lead or mercury. Hence the hydroxide of copper dissolves in
solutions of neutral cupric salts. The cupric salts are generally blue or
green, because cupric hydroxide itself is coloured. But some of the
salts in the anhydrous state are colourless.[10]
[412]
The commonest normal salt is blue vitriol—i.e. the normal cupric
sulphate. It generally contains five molecules of water of crystallisation,
CuSO4,5H2O. It forms the product of the action of strong sulphuric
acid on copper, sulphurous anhydride being evolved. The same
salt is obtained in practice by carefully roasting sulphuretted ores of
copper, and also by the action of water holding oxygen in solution on
them: CuS + O4 = CuSO4. This salt forms a by-product, obtained in
gold refineries, when the silver is precipitated from the sulphuric acid
solution by means of copper. It is also obtained by pouring dilute
sulphuric acid over sheet copper in the presence of air, or by heating
cupric oxide or carbonate in sulphuric acid. The crystals of this salt
belong to the triclinic system, have a specific gravity of 2·19, are of a
beautiful blue colour, and give a solution of the same colour. 100
parts of water at 0° dissolve 15, at 25° 23, and at 100° about 45 parts
of cupric sulphate, CuSO4.[10 bis] At 100° this salt loses a portion of its[413]
water of crystallisation, which it only parts with entirely at a high
temperature (220°) and then gives a white powder of the anhydrous
sulphate; and the latter, on further calcination, loses the elements of
sulphuric anhydride, leaving cupric oxide, like all the cupric salts. The
anhydrous (colourless) cupric sulphate is sometimes used for absorbing
water; it turns blue in the process. It offers the advantage that it
retains both hydrochloric acid and water, but not carbonic anhydride.[11]
Cupric sulphate is used for steeping seed corn; this is said to prevent
the growth of certain parasites on the plants. In the arts a considerable
quantity of cupric sulphate is also used in the preparation of other
copper salts—for instance, of certain pigments[11 bis]—and a particularly[414]
large quantity is used in the galvanoplastic process, which consists in
the deposition of copper from a solution of cupric sulphate by the
action of a galvanic current, when the metallic copper is deposited
on the negative pole and takes the shape of the latter. The description
of the processes of galvanoplastic art introduced by Jacobi
in St. Petersburg forms a part of applied physics, and will not be
touched on here, and we will only mention that, although first introduced
for small articles, it is now used for such articles as type moulds
(clichés), for maps, prints, &c., and also for large statues, and for the
deposition of iron, zinc, nickel, gold, silver, &c. on other metals and
materials. The beginning of the application of the galvanic current to
the practical extraction of metals from solutions has also been established,
especially since the dynamo-electric machines of Gramme,
Siemens, and others have rendered it possible to cheaply convert the
mechanical motion of the steam engine into an electric current. It is
to be expected that the application of the electric current, which has
long since given such important results in chemistry, will, in the near
future, play an important part in technical processes, the example being
shown by electric lighting.
The alloys of copper with certain metals, and especially with zinc
and tin, are easily formed by directly melting the metals together.
They are easily cast into moulds, forged, and worked like copper,
whilst they are much more durable in the air, and are therefore frequently
used in the arts. Even the ancients used exclusively alloys
of copper, and not pure copper, but its alloys with tin or different
kinds of bronze (Chapter XVIII., Note 35). The alloys of copper with
zinc are called brass or ‘yellow metal.’ Brass contains about 32 p.c. of
zinc; generally, however, it does not contain more than 65 p.c. of
copper. The remainder is composed of lead and tin, which usually
occur, although in small quantities, in brass. Yellow metal contains
about 40 p.c. of zinc.[12] The addition of zinc to copper changes the[415]
colour of the latter to a considerable degree; with a certain amount of
zinc the colour of the copper becomes yellow, and with a still larger
proportion of zinc an alloy is formed which has a greenish tint. In those
alloys of zinc and copper which contain a larger amount of zinc than
of copper, the yellow colour disappears and is replaced by a greyish
colour. But when the amount of zinc is diminished to about 20 p.c.,
the alloy is red and hard, and is called ‘tombac.’ A contraction takes
place in alloying copper with zinc, so that the volume of the alloy is
less than that of either metal individually. The zinc volatilises on
prolonged heating at a high temperature and the excess of metallic
copper remains behind. When heated in the air, the zinc oxidises
before the copper, so that all the zinc alloyed with copper may be
removed from the copper by this means. An important property of
brass containing about 30 p.c. of zinc is that it is soft and malleable in
the cold, but becomes somewhat brittle when heated. We may also
mention that ordinary copper coins contain, in order to render them
hard, tin, zinc, and iron (Cu = 95 p.c.); that it is now customary to add
a small amount of phosphorus to copper and bronze, for the same purpose;
and also that copper is added to silver and gold in coining, &c.
to render it hard; moreover, in Germany, Switzerland, and Belgium,
and other countries, a silver-white alloy (melchior, German silver, &c.),
for base coinage and other purposes, is prepared from brass and nickel
(from 10 to 20 p.c. of nickel; 20 to 30 p.c. zinc: 50 to 70 p.c. copper),
or directly from copper and nickel, or, more rarely, from an alloy containing
silver, nickel, and copper.[12 bis]
Copper, in its cuprous compounds, is so analogous to silver, that[416]
were there no cupric compounds, or if silver gave stable compounds
of the higher oxide, AgO, the resemblance would be as close as that
between chlorine and bromine or zinc and cadmium; but silver
compounds corresponding to AgO are quite unknown. Although
silver peroxide—which was regarded as AgO, but which Berthelot
(1880) recognised as the sesquioxide Ag2O3—is known, still it does
not form any true salts, and consequently cannot be placed along
with cupric oxide. In distinction to copper, silver as a metal does
not oxidise under the influence of heat; and its oxides, Ag2O and
Ag2O3, easily lose oxygen (see Note 8 tri). Silver does not oxidise in
air at the ordinary pressure, and is therefore classed among the
so-called noble metals. It has a white colour, which is much purer
than that of any other known metal, especially when the metal is chemically
pure. In the arts silver is always used alloyed, because chemically-pure
silver is so soft that it wears exceedingly easily, whilst when
fused with a small amount of copper, it becomes very hard, without
losing its colour.[13]
[417]
[418]
Silver occurs in nature, both in a native state and in certain compounds.
Native silver, however, is of rather rare occurrence. A far
greater quantity of silver occurs in combination with sulphur, and
especially in the form of silver sulphide, Ag2S, with lead sulphide
or copper sulphide, or the ores of various other metals. The largest
amount of silver is extracted from the lead in which it occurs. If this
lead be calcined in the presence of air, it oxidises, and the resultant
lead oxide, PbO (‘litharge’ or ‘silberglätte,’ as it is called), melts into
a mobile liquid, which is easily removed. The silver remains in an
unoxidised metallic state.[14] This process is called cupellation.
[419]
Commercial silver generally contains copper, and, more rarely, other
metallic impurities also. Chemically pure silver is obtained either by
cupellation or by subjecting ordinary silver to the following treatment.
The silver is first dissolved in nitric acid, which converts it and the
copper into nitrates, Cu(NO3)2 and AgNO3; hydrochloric acid is then
added to the resultant solution (green, owing to the presence of the
cupric salt), which is considerably diluted with water in order to retain
the lead chloride in solution if the silver contained lead. The copper
and many other metals remain in solution, whilst the silver is precipitated
as silver chloride. The precipitate is allowed to settle, and the
liquid is decanted off; the precipitate is then washed and fused with
sodium carbonate. A double decomposition then takes place, sodium
chloride and silver carbonate being formed; but the latter decomposes
into metallic silver, because the silver oxide is decomposed by heat:
Ag2CO3 = Ag2 + O + CO2. The silver chloride may also be mixed
with metallic zinc, sulphuric acid, and water, and left for some time,
when the zinc removes the chlorine from the silver chloride and precipitates
the silver as a powder. This finely-divided silver is called
‘molecular silver.’[15]
[420]
Chemically-pure silver has an exceeding pure white colour, and a
specific gravity of 10·5. Solid silver is lighter than the molten metal,
and therefore a piece of silver floats on the latter. The fusing-point
of silver is about 950° C., and at the high temperature attained
by the combustion of detonating gas it volatilises.[16] By employing
silver reduced from silver chloride by milk sugar and caustic potash,
and distilling it, Stas obtained silver purer than that obtained by any
other means; in fact, this was perfectly pure silver. The vapour of
silver has a very beautiful green colour, which is seen when a silver
wire is placed in an oxyhydrogen flame.[17]
It has long been known (Wöhler) that when nitrate of silver,
AgNO3, reacts as an oxidising agent upon citrates and tartrates, it is
able under certain conditions to give either a salt of suboxide of silver
(see Note 19) or a red solution, or to give a precipitate of metallic
silver reduced at the expense of the organic substances. In 1889 Carey
Lea, in his researches on this class of reactions, showed that soluble[421]
silver is here formed, which he called allotropic silver. It may be
obtained by taking 200 c.c. of a 10 per cent. solution of AgNO3 and
quickly adding a mixture (neutralised with NaHO) of 200 c.c. of a
30 per cent. solution of FeSO4 and 200 c.c. of a 40 per cent. solution
of sodium citrate. A lilac precipitate is obtained, which is collected
on a filter (the precipitate becomes blue) and washed with a solution of
NH4NO3. It then becomes soluble in pure water, forming a red
perfectly transparent solution from which the dissolved silver is precipitated
on the addition of many soluble foreign bodies. Some of the
latter—for instance, NH4NO3, alkaline sulphates, nitrates, and citrates—give
a precipitate which re-dissolves in pure water, whilst others—for
instance, MgSO4, FeSO4, K2Cr2O7, AgNO3, Ba(NO3)2 and many others—convert
the precipitated silver into a new variety, which, although no
longer soluble in water, regains its solubility in a solution of borax
and is soluble in ammonia. Both the soluble and insoluble silver are
rapidly converted into the ordinary grey-metallic variety by sulphuric
acid, although nothing is given off in the reaction; the same change
takes place on ignition, but in this case CO2 is disengaged; the latter
is formed from the organic substances which remain (to the amount of
3 per cent.) in the modified silver (they are not removed by soaking in
alcohol or water). If the precipitated silver be slightly washed and
laid in a smooth thin layer on paper or glass, it is seen that the soluble
variety is red when moist and a fine blue colour when dry, whilst the insoluble
variety has a blue reflex. Besides these, under special conditions[18][422]
a golden yellow variety may be obtained, which gives a brilliant golden
yellow coating on glass; but it is easily converted into the ordinary
grey-metallic state by friction or trituration. There is no doubt[18 bis]
that there is the same relation between ordinary silver which is perfectly
insoluble in water and the varieties of silver obtained by Carey
Lea[18 tri] as there is between quartz and soluble silica or between[423]
CuS and As2S2 in their ordinary insoluble forms and in the state of
the colloid solution of their hydrosols (see Chapter I., Note 57, and
Chapter XVII., Note 25 bis). Here, however, an important step in
advance has been made in this respect, that we are dealing with the
solution of a simple body, and moreover of a metal—i.e. of a particularly
characteristic state of matter. And as boron, gold, and certain
other simple bodies have already been obtained in a soluble (colloid)
form, and as numerous organic compounds (albuminous substances,
gum, cellulose, starch, &c.) and inorganic substances are also known in
this form, it might be said that the colloid state (of hydrogels and hydrosols)
can be acquired, if not by every substance, at all events by substances
of most varied chemical character under particular conditions
of formation from solutions. And this being the case, we may hope
that a further study of soluble colloid compounds, which apparently
present various transitions towards emulsions, may throw a new light
upon the complex question of solutions, which forms one of the problems
of the present epoch of chemical science. Moreover, we may remark that
Spring (1890) clearly proved the colloid state of soluble silver by means
of dialysis as it did not pass through the membrane.
As regards the capacity of silver for chemical reactions, it is
remarkable for its small capacity for combination with oxygen and for
its considerable energy of combination with sulphur, iodine, and certain
kindred non-metals. Silver does not oxidise at any temperature,
and its oxide, Ag2O, is decomposed by heat. It is also a very important
fact that silver is not oxidised by oxygen either in the presence of
alkalis, even at exceedingly high temperatures, or in the presence of
acids—at least, of dilute acids—which properties render it a very
important metal in chemical industry for the fusion of alkalis, and also
for many purposes in everyday life; for instance, for making spoons,
salt-cellars, &c. Ozone, however, oxidises it. Of all acids nitric acid
has the greatest action on silver. The reaction is accompanied by the
formation of oxides of nitrogen and silver nitrate, AgNO3, which
dissolves in water and does not, therefore, hinder the further action of
the acid on the metal. The halogen acids, especially hydriodic acid,
act on silver, hydrogen being evolved; but this action soon stops,
owing to the halogen compounds of silver being insoluble in water and
only very slightly soluble in acids; they therefore preserve the remaining
mass of metal from the further action of the acid; in consequence of
this the action of the halogen acids is only distinctly seen with finely-divided
silver. Sulphuric acid acts on silver in the same manner that
it does on copper, only it must be concentrated and at a higher
temperature. Sulphurous anhydride, and not hydrogen, is then evolved,[424]
but there is no action at the ordinary temperature, even in the presence
of air. Among the various salts, sodium chloride (in the presence of
moisture, air, and carbonic acid) and potassium cyanide (in the presence
of air) act on silver more decidedly than any others, converting it respectively
into silver chloride and a double cyanide.
Although silver does not directly combine with oxygen, still three
different grades of combination with oxygen may be obtained indirectly
from the salts of silver. They are all, however, unstable, and
decompose into oxygen and metallic silver when ignited. These three
oxides of silver have the following composition: silver suboxide,
Ag4O,[19] corresponding with the (little investigated) suboxides of the
alkali metals; silver oxide, Ag2O, corresponding with the oxides of the
alkali metals and the ordinary salts of silver, AgX; and silver peroxide,
AgO,[19 bis] or, judging from Berthelot's researches, Ag2O3. Silver oxide
is obtained as a brown precipitate (which when dried does not contain
water) by adding potassium hydroxide to a solution of a silver salt—for
example, of silver nitrate. The precipitate formed seems, however,[425]
to be an hydroxide, AgHO, i.e. AgNO3 + KHO = KNO3 + AgHO,
and the formation of the anhydrous oxide, 2AgHO = Ag2O + H2O,
may be compared with the formation of the anhydrous cupric oxide by
the action of potassium hydroxide on hot cupric solutions. Silver
hydroxide decomposes into water and silver oxide, even at low
temperatures; at least, the hydroxide no longer exists at 60°, but
forms the anhydrous oxide, Ag2O.[19 tri] Silver oxide is almost
insoluble in water; but, nevertheless, it is undoubtedly a rather
powerful basic oxide, because it displaces the oxides of many metals
from their soluble salts, and saturates such acids as nitric acid,
forming with them neutral salts, which do not act on litmus paper.[20]
Undoubtedly water dissolves a small quantity of silver oxide,
which explains the possibility of its action on solutions of salts—for
example, on solutions of cupric salts. Water in which silver oxide
is shaken up has a distinctly alkaline reaction. The oxide is distinguished
by its great instability when heated, so that it loses all its
oxygen when slightly heated. Hydrogen reduces it at about 80°.[20 bis]
The feebleness of the affinity of silver for oxygen is shown by the fact
that silver oxide decomposes under the action of light, so that it must be
kept in opaque vessels. The silver salts are colourless and decompose
when heated, leaving metallic silver if the elements of the acid are
volatile.[20 tri] They have a peculiar metallic taste, and are exceedingly
poisonous; the majority of them are acted on by light, especially in
the presence of organic substances, which are then oxidised. The
alkaline carbonates give a white precipitate of silver carbonate,
Ag2CO3, which is insoluble in water, but soluble in ammonia and
ammonium carbonate. Aqueous ammonia, added to a solution of a
normal silver salt, first acts like potassium hydroxide, but the precipitate
dissolves in an excess of the reagent, like the precipitate of cupric[426]
hydroxide.[21] Silver oxalate and the halogen compounds of silver are
insoluble in water; hydrochloric acid and soluble chlorides give,
as already repeatedly observed, a white precipitate of silver chloride
in solutions of silver salts. Potassium iodide gives a yellowish
precipitate of silver iodide. Zinc separates all the silver in a metallic
form from solutions of silver salts. Many other metals and reducing
agents—for example, organic substances—also reduce silver from the
solutions of its salts.
Silver nitrate, AgNO3, is known by the name of lunar caustic
(or lapis infernalis); it is obtained by dissolving metallic silver
in nitric acid. If the silver be impure, the resultant solution will
contain a mixture of the nitrates of copper and silver. If this mixture
be evaporated to dryness and the residue carefully fused at an
incipient red heat, all the cupric nitrate is decomposed, whilst the
greater part of the silver nitrate remains unchanged. On treating
the fused mass with water the latter is dissolved, whilst the cupric
oxide remains insoluble. If a certain amount of silver oxide be added
to the solution containing the nitrates of silver and copper, it displaces
all the cupric oxide. In this case it is of course not necessary to take
pure silver oxide, but only to pour off some of the solution and to add
potassium hydroxide to one portion, and to mix the resultant precipitate
of the hydroxides, Cu(OH)2 and AgOH, with the remaining
portion.[22] By these methods all the copper can be easily removed and[427]
pure silver nitrate obtained (its solution is colourless, while the presence
of Cu renders it blue), which may be ultimately purified by crystallisation.
It crystallises in colourless transparent prismatic plates, which
are not acted on by air. They are anhydrous. Its sp. gr. is 4·34; it
dissolves in half its weight of water at the ordinary temperature.[22 bis]
The pure salt is not acted on by light, but it easily acts in an oxidising
manner on the majority of organic substances, which it generally
blackens. This is due to the fact that the organic substance is oxidised
by the silver nitrate, which is reduced to metallic silver; the latter is
thus obtained in a finely-divided state, which causes the black stain.
This peculiarity is taken advantage of for marking linen. Silver nitrate
is for the same reason used for cauterising wounds and various growths
on the body. Here again it acts by virtue of its oxidising capacity in
destroying the organic matter, which it oxidises, as is seen from the
separation of a coating of black metallic powdery silver from the part
cauterised.[22 tri] From the description of the preparation of silver nitrate
it will have been seen that this salt, which fuses at 218°, does not[428]
decompose at an incipient red heat; when cast into sticks it is usually
employed for cauterising. On further heating, the fused salt undergoes
decomposition, first forming silver nitrite and then metallic silver.
With ammonia, silver nitrate forms, on evaporation of the solution,
colourless crystals containing AgNO3,2HN3 (Marignac). In general
the salts of silver, like cuprous, cupric, zinc, &c. salts, are able to give
several compounds with ammonia; for example, silver nitrate in a dry
state absorbs three molecules (Rose). The ammonia is generally easily
expelled from these compounds by the action of heat.
Nitrate of silver easily forms double salts like AgNO32NaNO3 and
AgNO3KNO3. Silver nitrate under the action of water and a halogen
gives nitric acid (see Vol. I. p. 280, formation of N2O5), a halogen salt of
silver, and a silver salt of an oxygen acid of the halogen. Thus, for
example, a solution of chlorine in water, when mixed with a solution of
silver nitrate, gives silver chloride and chlorate. It is here evident that
the reaction of the silver nitrate is identical with the reaction of the
caustic alkalis, as the nitric acid is all set free and the silver oxide only
reacts in exactly the same way in which aqueous potash acts on free
chlorine. Hence the reaction may be expressed in the following
manner: 6AgNO3 + 3Cl2 + 3H2O = 5AgCl + AgClO3 + 6NHO3.
Silver nitrate, like the nitrates of the alkalis, does not contain any
water of crystallisation. Moreover the other salts of silver almost
always separate out without any water of crystallisation. The silver
salts are further characterised by the fact that they give neither
basic nor acid salts, owing to which the formation of silver salts
generally forms the means of determining the true composition of
acids—thus, to any acid HnX there corresponds a salt AgnX—for
instance, Ag3PO4 (Chapter XIX., Note 15).
Silver gives insoluble and exceedingly stable compounds with the
halogens. They are obtained by double decomposition with great
facility whenever a silver salt comes in contact with halogen salts.
Solutions of nitrate, sulphate, and all other kindred salts of silver give
a precipitate of silver chloride or iodide in solutions of chlorides and
iodides and of the halogen acids, because the halogen salts of silver are
insoluble both in water[23] and in other acids. Silver chloride, AgCl, is[429]
then obtained as a white flocculent precipitate, silver bromide forms a
yellowish precipitate, and silver iodide has a very distinct yellow
colour. These halogen compounds sometimes occur in nature; they
are formed by a dry method—by the action of halogen compounds on
silver compounds, especially under the influence of heat. Silver chloride
easily fuses at 451° on cooling from a molten state; it forms
a somewhat soft horn-like mass which can be cut with a knife
and is known as horn silver. It volatilises at a higher temperature.
Its ammoniacal solution, on the evaporation of the ammonia,
deposits crystalline chloride of silver, in octahedra. Bromide and
iodide of silver also appear in forms of the regular system, so that in
this respect the halogen salts of silver resemble the halogen salts of the
alkali metals.[24]
[430]
[431]
Silver chloride may be decomposed, with the separation of silver
oxide, by heating it with a solution of an alkali, and if an organic
substance be added to the alkali the chloride can easily be reduced o
metallic silver, the silver oxide being reduced in the oxidation of the
organic substance. Iron, zinc, and many other metals reduce silver
chloride in the presence of water. Cuprous and mercurous chlorides
and many organic substances are also able to reduce the silver from
chloride of silver. This shows the rather easy decomposability of the
halogen compounds of silver. Silver iodide is much more stable in this
respect than the chloride. The same is also observed with respect to
the action of light upon moist AgCl. White silver chloride soon acquires
a violet colour when exposed to the action of light, and especially
under the direct action of the sun's rays. After being acted upon
by light it is no longer entirely soluble in ammonia, but leaves
metallic silver undissolved, from which it might be assumed that the
action of light consisted in the decomposition of the silver chloride
into chlorine and metallic silver and in fact the silver chloride becomes
in time darker and darker. Silver bromide and iodide are much
more slowly acted on by light, and, according to certain observations,
when pure they are even quite unacted on; at least they do not change
in weight,[24 bis] so that if they are acted on by light, the change they
undergo must be one of a change in the structure of their parts and not
of decomposition, as it is in silver chloride. The silver chloride under
the action of light changes in weight, which indicates the formation of
a volatile product, and the deposition of metallic silver on dissolving
in ammonia shows the loss of chlorine. The change does actually
occur under the action of light, but the decomposition does not go
as far as into chlorine and silver, but only to the formation of a subchloride
of silver, Ag2Cl, which is of a brown colour and is easily decomposed
into metallic silver and silver chloride, Ag2Cl = AgCl + Ag.
This change of the chemical composition and structure of the halogen
salts of silver under the action of light forms the basis of photography,
because the halogen compounds of silver, after having been exposed to
light, give a precipitate of finely-divided silver, of a black colour,
when treated with reducing agents.[25]
[432]
The insolubility of the halogen compounds of silver forms the
basis of many methods used in practical chemistry. Thus by means of
this reaction it is possible to obtain salts of other acids from a halogen
salt of a given metal, for instance, RCl2 + 2AgNO3 = R(NO3)2 + 2AgCl.
The formation of the halogen compounds of silver is very frequently
used in the investigation of organic substances; for example, if any
product of metalepsis containing iodine or chlorine be heated with a
silver salt or silver oxide, the silver combines with the halogen and
gives a halogen salt, whilst the elements previously combined with the
silver replace the halogen. For instance, ethylene dibromide, C2H4Br2,
is transformed into ethylene diacetate, C2H4(C2H3O2)2, and silver[433]
bromide by heating it with silver acetate, 2C2H3O2Ag. The insolubility
of the halogen compounds of silver is still more frequently taken advantage
of in determining the amount of silver and halogen in a given
solution. If it is required, for instance, to determine the quantity of
chlorine present in the form of a metallic chloride in a given solution,
a solution of silver nitrate is added to it so long as it gives a precipitate.
On shaking or stirring the liquid, the silver chloride easily
settles in the form of heavy flakes. It is possible in this way to
precipitate the whole of the chlorine from a solution, without adding
an excess of silver nitrate, since it can be easily seen whether the
addition of a fresh quantity of silver nitrate produces a precipitate in
the clear liquid. In this manner it is possible to add to a solution
containing chlorine, as much silver as is required for its entire precipitation,
and to calculate the amount of chlorine previously in solution
from the amount of the solution of silver nitrate consumed, if the
quantity of silver nitrate in this solution has been previously determined.[25 bis]
The atomic proportions and preliminary experiments with
a pure salt—for example, with sodium chloride—will give the amount
of chlorine from the quantity of silver nitrate. Details of these
methods will be found in works on analytical chemistry.[25 tri]
[434]
Accurate experiments, and more especially the researches of
Stas at Brussels, show the proportion in which silver reacts with
metallic chlorides. These researches have led to the determination
of the combining weights of silver, sodium, potassium, chlorine,
bromine, iodine, and other elements, and are distinguished for their
model exactitude, and we will therefore describe them in some detail.
As sodium chloride is the chloride most generally used for the precipitation
of silver, since it can most easily be obtained in a pure state,
we will here cite the quantitative observations made by Stas for showing
the co-relation between the quantities of chloride of sodium and
silver which react together. In order to obtain perfectly pure sodium[435]
chloride, he took pure rock salt, containing only a small quantity of
magnesium and calcium compounds and a small amount of potassium
salts. This salt was dissolved in water, and the saturated solution
evaporated by boiling. The sodium chloride separated out during the
boiling, and the mother liquor containing the impurities was poured
off. Alcohol of 65 p.c. strength and platinic chloride were added
to the resultant salt, in order to precipitate all the potassium and
a certain part of the sodium salts. The resultant alcoholic solution,
containing the sodium and platinum chlorides, was then mixed with a
solution of pure ammonium chloride in order to remove the platinic
chloride. After this precipitation, the solution was evaporated in a
platinum retort, and then separate portions of this purified sodium
chloride were collected as they crystallised. The same salt was prepared
from sodium sulphate, tartrate, nitrate, and from the platinochloride,
in order to have sodium chloride prepared by different methods
and from different sources, and in this manner ten samples of sodium
chloride thus prepared were purified and investigated in their relation
to silver. After being dried, weighed quantities of all ten samples
of sodium chloride were dissolved in water and mixed with a solution
in nitric acid of a weighed quantity of perfectly pure silver. A
slightly greater quantity of silver was taken than would be required
for the decomposition of the sodium chloride, and when, after pouring
in all the silver solution, the silver chloride had settled, the
amount of silver remaining in excess was determined by means of a
solution of sodium chloride of known strength. This solution of
sodium chloride was added so long as it formed a precipitate. In this
manner Stas determined how many parts of sodium chloride correspond
to 100 parts by weight of silver. The result of ten determinations
was that for the entire precipitation of 100 parts of silver,
from 54·2060 to 54·2093 parts of sodium chloride were required. The
difference is so inconsiderable that it has no perceptible influence
on the subsequent calculations. The mean of ten experiments was
that 100 parts of silver react with 54·2078 parts of sodium chloride.
In order to learn from this the relation between the chlorine and
silver, it was necessary to determine the quantity of chlorine contained in
54·2078 parts of sodium chloride, or, what is the same thing, the quantity
of chlorine which combines with 100 parts of silver. For this purpose
Stas made a series of observations on the quantity of silver chloride
obtained from 100 parts of silver. Four syntheses were made by him
for this purpose. The first synthesis consisted in the formation of
silver chloride by the action of chlorine on silver at a red heat. This
experiment showed that 100 parts of silver give 132·841, 132·843 and[436]
132·843 of silver chloride. The second method consisted in dissolving
a given quantity of silver in nitric acid and precipitating it by means
of gaseous hydrochloric acid passed over the surface of the liquid; the
resultant mass was evaporated in the dark to drive off the nitric acid
and excess of hydrochloric acid, and the remaining silver chloride was
fused first in an atmosphere of hydrochloric acid gas and then in air.
In this process the silver chloride was not washed, and therefore there
could be no loss from solution. Two experiments made by this
method showed that 100 parts of silver give 132·849 and 132·846
parts of silver chloride. A third series of determinations was also
made by precipitating a solution of silver nitrate with a certain
excess of gaseous hydrochloric acid. The amount of silver chloride
obtained was altogether 132·848. Lastly, a fourth determination was
made by precipitating dissolved silver with a solution of ammonium
chloride, when it was found that a considerable amount of silver
(0·3175) had passed into solution in the washing; for 100 parts
of silver there was obtained altogether 132·8417 of silver chloride.
Thus from the mean of seven determinations it appears that 100
parts of silver give 132·8445 parts of silver chloride—that is, that
32·8445 parts of chlorine are able to combine with 100 parts of
silver and with that quantity of sodium which is contained in
54·2078 parts of sodium chloride. These observations show that
32·8445 parts of chlorine combine with 100 parts of silver and
with 21·3633 parts of sodium. From these figures expressing the
relation between the combining weights of chlorine, silver, and sodium,
it would be possible to determine their atomic weights—that is, the
combining quantity of these elements with respect to one part by
weight of hydrogen or 16 parts of oxygen, if there existed a series of
similarly accurate determinations for the reactions between hydrogen
or oxygen and one of these elements—chlorine, sodium, or silver. If
we determine the quantity of silver chloride which is obtained from
silver chlorate, AgClO3, we shall know the relation between the
combining weights of silver chloride and oxygen, so that, taking the
quantity of oxygen as a constant magnitude, we can learn from this
reaction the combining weight of silver chloride, and from the preceding
numbers the combining weights of chlorine and silver. For this
purpose it was first necessary to obtain pure silver chlorate. This
Stas did by acting on silver oxide or carbonate, suspended in water,
with gaseous chlorine.[26]
[437]
The decomposition of the silver chlorate thus obtained was accomplished
by the action of a solution of sulphurous anhydride on
it. The salt was first fused by carefully heating it at 243°. The solution
of sulphurous anhydride used was one saturated at 0°. Sulphurous
anhydride in dilute solutions is oxidised at the expense of silver
chlorate, even at low temperatures, with great ease if the liquid be
continually shaken, sulphuric acid and silver chloride being formed:
AgClO3 + 3SO2 + 3H2O = AgCl + 3H2SO4. After decomposition, the
resultant liquid was evaporated, and the residue of silver chloride
weighed. Thus the process consisted in taking a known weight of
silver chlorate, converting it into silver chloride, and determining
the weight of the latter. The analysis conducted in this manner gave
the following results, which, like the preceding, designate the weight
in a vacuum calculated from the weights obtained in air: In the
first experiment it appeared that 138·7890 grams of silver chlorate
gave 103·9795 parts of silver chloride, and in the second experiment[438]
that 259·5287 grains of chlorate gave 194·44515 grams of silver
chloride, and after fusion 194·4435 grams. The mean result of both
experiments, converted into percentages, shows that 100 parts of silver
chlorate contain 74·9205 of silver chloride and 25·0795 parts of oxygen.
From this it is possible to calculate the combining weight of silver
chloride, because in the decomposition of silver chlorate there are
obtained three atoms of oxygen and one molecule of silver
chloride: AgClO3 = AgCl + 3O. Taking the weight of an atom
of oxygen to be 16, we find from the mean result that the equivalent
weight of silver chloride is equal to 143·395. Thus if O = 16,
AgCl = 143·395, and as the preceding experiments show that silver
chloride contains 32·8445 parts of chlorine per 100 parts of silver,
the weight of the atom of silver[26 bis] must be 107·94 and that
of chlorine 35·45. The weight of the atom of sodium is determined
from the fact that 21·3633 parts of sodium chloride combine with
32·8445 parts of chlorine; consequently Na = 23·05. This conclusion,
arrived at by the analysis of silver chlorate, was verified by means
of the analysis of potassium chlorate by decomposing it by heat
and determining the weight of the potassium chloride formed, and also
by effecting the same decomposition by igniting the chlorate in a
stream of hydrochloric acid. The combining weight of potassium
chloride was thus determined, and another series of determinations
confirmed the relation between chlorine, potassium, and silver, in the
same manner as the relation between sodium, chlorine, and silver was
determined above. Consequently, the combining weights of sodium,
chlorine, and potassium could be deduced by combining these data with
the analysis of silver chlorate and the synthesis of silver chloride. The
agreement between the results showed that the determinations made
by the last method were perfectly correct, and did not depend in any
considerable degree on the methods which were employed in the preceding
determinations, as the combining weights of chlorine and silver
obtained were the same as before. There was naturally a difference,
but so small a one that it undoubtedly depended on the errors incidental
to every process of weighing and experiment. The atomic weight
of silver was also determined by Stas by means of the synthesis of
silver sulphide and the analysis of silver sulphate. The combining
weight obtained by this method was 107·920. The synthesis of silver
iodide and the analysis of silver iodate gave the figure 107·928. The[439]
synthesis of silver bromide with the analysis of silver bromate gave the
figure 107·921. The synthesis of silver chloride and the analysis of
silver chlorate gave a mean result of 107·937. Hence there is no
doubt that the combining weight of silver is at least as much as 107·9—greater
than 107·90 and less than 107·95, and probably equal to the
mean = 107·92. Stas determined the combining weights of many other
elements in this manner, such as lithium, potassium, sodium, bromine,
chlorine, iodine, and also nitrogen, for the determination of the
amount of silver nitrate obtained from a given amount of silver
gives directly the combining weight of nitrogen. Taking that
of oxygen as 16, he obtained the following combining weights
for these elements: nitrogen 14·04, silver 107·93, chlorine 35·46,
bromine 79·95, iodine 126·85, lithium 7·02, sodium 23·04, potassium
39·15. These figures differ slightly from those which are usually
employed in chemical investigations. They must be regarded as the
result of the best observations, whilst the figures usually used in
practical chemistry are only approximate—are, so to speak, round
numbers for the atomic weights which differ so little from the exact
figures (for instance, for Ag 108 instead of 107·92, for Na 23 instead
of 23·04) that in ordinary determinations and calculations the
difference falls within the limits of experimental error inseparable from
such determinations.
The exhaustive investigations conducted by Stas on the atomic
weights of the above-named elements have great significance in
the solution of the problem as to whether the atomic weights of the
elements can be expressed in whole numbers if the unit taken be the
atomic weight of hydrogen. Prout, at the beginning of this century,
stated that this was the case, and held that the atomic weights of the
elements are multiples of the atomic weight of hydrogen. The subsequent
determinations of Berzelius, Penny, Marchand, Marignac, Dumas,
and more especially of Stas, proved this conclusion to be untenable;
since a whole series of elements proved to have fractional atomic
weights—for example, chlorine, about 35·5. On account of this,
Marignac and Dumas stated that the atomic weights of the elements
are expressed in relation to hydrogen, either by whole numbers
or by numbers with simple fractions of the magnitudes ½ and ¼. But
Stas's researches refute this supposition also. Even between the combining
weight of hydrogen and oxygen, there is not, so far as is yet
known, that simple relation which is required by Prout's hypothesis,[27][440]
i.e., taking O = 16, the atomic weight of hydrogen is equal not to 1 but
to a greater number somewhere between 1·002 and 1·008 or mean[441]
1·005. Such a conclusion arrived at by direct experiment cannot but
be regarded as having greater weight than Prout's supposition
(hypothesis) that the atomic weights of the elements are in multiple
proportion to each other, which would give reason for surmising (but not
asserting) a complexity of nature in the elements, and their common
origin from a single primary material, and for expecting their
mutual conversion into each other. All such ideas and hopes must[442]
now, thanks more especially to Stas, be placed in a region void of any
experimental support whatever, and therefore not subject to the discipline
of the positive data of science.
Among the platinum metals ruthenium, rhodium, and palladium,
by their atomic weights and properties, approach silver, just as iron
and its analogues (cobalt and nickel) approach copper in all respects.
Gold stands in exactly the same position in relation to the heavy
platinum metals, osmium, iridium, and platinum, as copper and
silver do to the two preceding series. The atomic weight of gold is
nearly equal to their atomic weights;[28] it is dense like these metals.
It also gives various grades of oxidation, which are feeble, both in
a basic and an acid sense. Whilst near to osmium, iridium, and platinum,
gold at the same time is able, like copper and silver, to form
compounds which answer to the type RX—that is, oxides of the composition
R2O. Cuprous chloride, CuCl, silver chloride, AgCl, and aurous
chloride, AuCl, are substances which are very much alike in their
physical and chemical properties.[28 bis] They are insoluble in water,
but dissolve in hydrochloric acid and ammonia, in potassium cyanide,[443]
sodium thiosulphate, &c. Just as copper forms a link between the iron
metals and zinc, and as silver unites the light platinum metals with
cadmium, so also gold presents a transition from the heavy platinum
metals to mercury. Copper gives saline compounds of the types CuX
and CuX2, silver of the type AgX, whilst gold, besides compounds of
the type AuX, very easily and most frequently forms those of the type
AuCl3. The compounds of this type frequently pass into those of the
lower type, just as PtX4 passes into PtX2, and the same is observable
in the elements which, in their atomic weights, follow gold. Mercury
gives HgX2 and HgX, thallium gives TlX3 and TlX, lead gives
PbX4 and PbX2. On the other hand, gold in a qualitative respect
differs from silver and copper in the extreme ease with which all its compounds
are reduced to metal by many means. This is not only accomplished
by many reducing agents, but also by the action of heat. Thus
its chlorides and oxides lose their chlorine and oxygen when heated,
and, if the temperature be sufficiently high, these elements are entirely
expelled and metallic gold alone remains. Its compounds, therefore,
act as oxidising agents.[29]
In nature gold occurs in the primary and chiefly in quartzose rocks,
and especially in quartz veins, as in the Urals (at Berezoffsk), in
Australia, and in California. The native gold is extracted from these
rocks by subjecting them to a mechanical treatment consisting of
crushing and washing.[29 bis] Nature has already accomplished a similar[444]
disintegration of the hard rocky matter containing gold.[30] These disintegrated
rocks, washed by rain and other water, have formed gold-bearing
deposits, which are known as alluvial gold deposits. Gold-bearing
soil is sometimes met with on the surface and sometimes under[445]
the upper soil, but more frequently along the banks of dried-up water-courses
and running streams. The sand of many rivers contains,
however, a very small amount of gold, which it is not profitable to
work; for example, that of the Alpine rivers contains 5 parts of gold
in 10,000,000 parts of sand. The richest gold deposits are those of
Siberia, especially in the southern parts of the Government of Yeniseisk,
the South Urals, Mexico, California, South Africa, and Australia,
and then the comparatively poorer alluvial deposits of many countries
(Hungary, the Alps, and Spain in Europe). The extraction of the
gold from alluvial deposits is based on the principle of levigation; the
earth is washed, while constantly agitated, by a stream of water,
which carries away the lighter portion of the earth, and leaves the
coarser particles of the rock and heavier particles of the gold, together
with certain substances which accompany it, in the washing apparatus.
The extraction of this washed gold only necessitates mechanical appliances,[31]
and it is not therefore surprising that gold was known to
savages and in the most remote period of history. It sometimes occurs
in crystals belonging to the regular system, but in the majority of cases[446]
in nuggets or grains of greater or less magnitude. It always contains
silver (from very small quantities up to 30 p.c., when it is called
‘electrum’) and certain other metals, among which lead and rhodium
are sometimes found.
The separation of the silver from gold is generally carried on with
great precision, as the presence of the silver in the gold does not
increase its value for exchange, and it can be substituted by other
less valuable metals, so that the extraction of the silver, as a precious
metal, from its alloy with gold, is a profitable operation. This
separation is conducted by different methods. Sometimes the argentiferous
gold is melted in crucibles, together with a mixture of common
salt and powdered bricks. The greater portion of the silver is thus
converted into the chloride, which fuses and is absorbed by the slags,
from which it may be extracted by the usual methods. The silver is
also extracted from gold by treating it with boiling sulphuric acid,
which does not act on the gold but dissolves the silver. But if the
alloy does not contain a large proportion of silver it cannot be extracted
by this method or at all events the separation will be imperfect, and
therefore a fresh amount of silver is added (by fusion) to the gold, in
such quantity that the alloy contains twice as much silver as gold.
The silver which is added is preferably such as contains gold, which is
very frequently the case. The alloy thus formed is poured in a thin
stream into water, by which means it is obtained in a granulated
form; it is then boiled with strong sulphuric acid, three parts of
acid being used to one part of alloy. The sulphuric acid extracts
all the silver without acting on the gold. It is best, however, to
pour off the first portion of the acid, which has dissolved the silver,
and then treat the residue of still imperfectly pure gold with a fresh
quantity of sulphuric acid. The gold is thus obtained in the form
of powder, which is washed with water until it is quite free from
silver. The silver is precipitated from the solution by means of
copper, so that cupric sulphate and metallic silver are obtained. This
process is carried out in many countries, as in Russia, at the Government
mints.
Gold is generally used alloyed with copper; since pure gold,
like pure silver, is very soft, and therefore soon worn away. In
assaying or determining the amount of pure gold in such an alloy
it is usual to add silver to the gold in order to make up an alloy
containing three parts of silver to one of gold (this is known as
quartation because the alloy contains ¼ of gold), and the resultant
alloy is treated with nitric acid. If the silver be not in excess over
the gold, it is not all dissolved by the nitric acid, and this is the reason[447]
for the quartation. The amount of pure gold (assay) is determined by
weighing the gold which remains after this treatment. English gold
(= 22 carats) coinage is composed of an alloy containing 91·66 p.c. of
gold, but for many articles gold is frequently used containing a larger
amount of foreign metals.
Pure gold may be obtained from gold alloys by dissolving in aqua
regia, and then adding ferrous sulphate to the solution or heating it
with a solution of oxalic acid. These deoxidising agents reduce the
gold, but not the other metals. The chlorine combined with the gold
then acts like free chlorine. The gold, thus reduced, is precipitated as
an exceedingly fine brown powder.[31 bis] It is then washed with water,
and fused with nitre or borax. Pure gold reflects a yellow light, and
in the form of very thin sheets (gold leaf), into which it can be
hammered and rolled,[31 tri] it transmits a bluish-green light. The
specific gravity of gold is about 19·5, the sp. gr. of gold coin is about
17·1. It fuses at 1090°—at a higher temperature than silver—and can
be drawn into exceedingly fine wires or hammered into thin sheets.
With its softness and ductility, gold is distinguished for its tenacity,
and a gold wire two millimetres thick breaks only under a load of 68
kilograms. Gold vaporises even at a furnace heat, and imparts a
greenish colour to a flame passing over it in a furnace. Gold alloys
with copper almost without changing its volume.[32] In its chemical[448]
aspect, gold presents, as is already seen from its general characteristics
given above, an example of the so-called noble metals—i.e. it is
incapable of being oxidised at any temperature, and its oxide is
decomposed when calcined. Only chlorine and bromine combine
directly with it at the ordinary temperature, but many other metals
and non-metals combine with it at a red heat—for example, sulphur,
phosphorus, and arsenic. Mercury dissolves it with great ease. It
dissolves in potassium cyanide in the presence of air; a mixture of
sulphuric acid with nitric acid dissolves it with the aid of heat,
although in small quantity. It is also soluble in aqua regia and in
selenic acid. Sulphuric, hydrochloric, nitric, and hydrofluoric acids
and the caustic alkalis do not act on gold, but a mixture of hydrochloric
acid with such oxidising agents as evolve chlorine naturally
dissolves it like aqua regia.[32 bis]
As regards the compounds of gold, they belong, as was said
above, to the types AuX3 and AuX. Auric chloride or gold trichloride,
AuCl3, which is formed when gold is dissolved in aqua regia,
belongs to the former and higher of these types. The solution of this
substance in water has a yellow colour, and it may be obtained pure by
evaporating the solution in aqua regia to dryness, but not to the point
of decomposition. If the evaporation proceed to the point of crystallisation,
a compound of gold chloride and hydrochloric acid, AuHCl4, is
obtained, like the allied compounds of platinum; but it easily parts
with the acid and leaves auric chloride, which fuses into a red-brown
liquid, and then solidifies to a crystalline mass. If dry chlorine be
passed over gold in powder it forms a mixture of aurous and auric
chlorides, but the aurous chloride is also decomposed by water into
gold and auric chloride. Auric chloride crystallises from its solutions
as AuCl3,2H2O, which easily loses water, and the dry chloride loses
two-thirds of its chlorine at 185°, forming aurous chloride, whilst[449]
above 300° the latter chloride also loses its chlorine and leaves
metallic gold. Auric chloride is the usual form in which gold occurs in
solutions, and in which its salts are used in the arts and for chemical
purposes. It is soluble in water, alcohol, and ether. Light has a reducing
action on these solutions, and after a time metallic gold is deposited
upon the sides of vessels containing the solution. Hydrogen when
nascent, and even in a gaseous form, reduces gold from this solution
to a metallic state. The reduction is more conveniently and usually
effected by ferrous sulphate, and in general by the action of ferrous
salts.[33]
If a solution of potassium hydroxide be added to a solution of auric
chloride, a precipitate is first formed, which re-dissolves in an excess of
the alkali. On being evaporated under the receiver of an air-pump,
this solution yields yellow crystals, which present the same composition
as the double salts AuMCl4, with the substitution of the chlorine by
oxygen—that is to say, potassium aurate, AuKO2, is formed in crystals
containing 3H2O. The solution has a distinctly alkaline reaction.
Auric oxide, Au2O3, separates when this alkaline solution is boiled with
an excess of sulphuric acid. But it then still retains some alkali; however,
it may be obtained in a pure state as a brown powder by
dissolving in nitric acid and diluting with water. The brown powder
decomposes below 250° into gold and oxygen. It is insoluble in water
and in many acids, but it dissolves in alkalis, which shows the acid
character of this oxide. An hydroxide, Au(OH)3 may be obtained as a
brown powder by adding magnesium oxide to a solution of auric chloride
and treating the resultant precipitate of magnesium aurate with
nitric acid. This hydroxide loses water at 100°, and gives auric oxide.[34]
[450]
The starting-point of the compounds of the type AuX[35] is gold
monochloride or aurous chloride, AuCl, which is formed, as mentioned
above, by heating auric chloride at 185°. Aurous chloride forms a
yellowish-white powder; this, when heated with water, is decomposed
into metallic gold and auric chloride, which passes into solution:
3AuCl = AuCl3 + 2Au. This decomposition is accelerated by the action
of light. Hence it is obvious that the compounds corresponding with
aurous oxide are comparatively unstable. But this only refers to the
simple compounds AuX; some of the complex compounds, on the
contrary, form the most stable compounds of gold. Such, for example,
is the cyanide of gold and potassium, AuK(CN)2. It is formed,
for instance, when finely-divided gold dissolves in the presence of
air in a solution of potassium cyanide: 4KCN + 2Au + H2O + O
= 2KAu(CN)2 + 2KHO (this reaction also proceeds with solid pieces
of gold, although very slowly). The same compound is formed in
solution when many compounds of gold are mixed with potassium
cyanide, because if a higher compound of gold be taken, it is reduced[451]
by the potassium cyanide into aurous oxide, which dissolves in potassium
cyanide and forms KAu(CN)2. This substance is soluble in
water, and gives a colourless solution, which can be kept for a long
time, and is employed in electro-gilding—that is, for coating other
metallic objects with a layer of gold, which is deposited if the object
be connected with the negative pole of a battery and the positive pole
consist of a gold plate. When an electric current is passed between
them, the gold from the latter will dissolve, whilst a coating of gold
from the solution will be deposited on the object.
[453]
APPENDIX I
AN ATTEMPT TO APPLY TO CHEMISTRY ONE OF THE
PRINCIPLES OF NEWTON'S NATURAL PHILOSOPHY
By PROFESSOR MENDELÉEFF
A LECTURE DELIVERED AT THE ROYAL INSTITUTION OF GREAT BRITAIN ON FRIDAY, MAY 31, 1889
Nature, inert to the eyes of the ancients, has been revealed to us as full of
life and activity. The conviction that motion pervaded all things, which was
first realised with respect to the stellar universe, has now extended to the
unseen world of atoms. No sooner had the human understanding denied to
the earth a fixed position and launched it along its path in space, than it was
sought to fix immovably the sun and the stars. But astronomy has demonstrated
that the sun moves with unswerving regularity through the star-set
universe at the rate of about 50 kilometres per second. Among the so-called
fixed stars are now discerned manifold changes and various orders of movement.
Light, heat, electricity—like sound—have been proved to be modes
of motion; to the realisation of this fact modern science is indebted for
powers which have been used with such brilliant success, and which have been
expounded so clearly at this lecture table by Faraday and by his successors.
As, in the imagination of Dante, the invisible air became peopled with spiritual
beings, so before the eyes of earnest investigators, and especially before those
of Clerk Maxwell, the invisible mass of gases became peopled with particles:
their rapid movements, their collisions, and impacts became so manifest that
it seemed almost possible to count the impacts and determine many of
the peculiarities or laws of their collisions. The fact of the existence of
these invisible motions may at once be made apparent by demonstrating the
difference in the rate of diffusion through porous bodies of the light and
rapidly moving atoms of hydrogen and the heavier and more sluggish particles
of air. Within the masses of liquid and of solid bodies we have been
forced to acknowledge the existence of persistent though limited motion of
their ultimate particles, for otherwise it would be impossible to explain, for
example, the celebrated experiments of Graham on diffusion through liquid
and colloidal substances. If there were, in our times, no belief in the[454]
molecular motion in solid bodies, could the famous Spring have hoped to
attain any result by mixing carefully-dried powders of potash, saltpetre and
sodium acetate, in order to produce, by pressure, a chemical reaction between
these substances through the interchange of their metals, and have derived,
for the conviction of the incredulous, a mixture of two hygroscopic though
solid salts—sodium nitrate and potassium acetate?
In these invisible and apparently chaotic movements, reaching from the
stars to the minutest atoms, there reigns, however, a harmonious order which
is commonly mistaken for complete rest, but which is really a consequence
of the conservation of that dynamic equilibrium which was first discerned
by the genius of Newton, and which has been traced by his successors in the
detailed analysis of the particular consequences of the great generalisation,
namely, relative immovability in the midst of universal and active movement.
But the unseen world of chemical changes is closely analogous to the
visible world of the heavenly bodies, since our atoms form distinct portions
of an invisible world, as planets, satellites, and comets form distinct portions
of the astronomer's universe; our atoms may therefore be compared to the
solar systems, or to the systems of double or of single stars: for example,
ammonia (NH3) may be represented in the simplest manner by supposing
the sun, nitrogen, surrounded by its planets of hydrogen; and common salt
(NaCl) may be looked on as a double star formed of sodium and chlorine.
Besides, now that the indestructibility of the elements has been acknowledged,
chemical changes cannot otherwise be explained than as changes of
motion, and the production by chemical reactions of galvanic currents, of
light, of heat, of pressure, or of steam power, demonstrates visibly that the
processes of chemical reaction are inevitably connected with enormous though
unseen displacements, originating in the movements of atoms in molecules.
Astronomers and natural philosophers, in studying the visible motions of the
heavenly bodies and of matter on the earth, have understood and have estimated
the value of this store of energy. But the chemist has had to pursue
a contrary course. Observing in the physical and mechanical phenomena
which accompany chemical reactions the quantity of energy manifested by
the atoms and molecules, he is constrained to acknowledge that within the
molecules there exist atoms in motion, endowed with an energy which, like
matter itself, is neither being created nor capable of being destroyed. Therefore,
in chemistry, we must seek dynamic equilibrium not only between the
molecules, but also in their midst among their component atoms. Many
conditions of such equilibrium have been determined, but much remains to be
done, and it is not uncommon, even in these days, to find that some chemists
forget that there is the possibility of motion in the interior of molecules, and
therefore represent them as being in a condition of death-like inactivity.
Chemical combinations take place with so much ease and rapidity,
possess so many special characteristics, and are so numerous, that their simplicity
and order were for a long time hidden from investigators. Sympathy,
relationship, all the caprices or all the fancifulness of human intercourse,
seemed to have found complete analogies in chemical combinations, but with
this difference, that the characteristics of the material substances—such as
silver, for example, or of any other body—remain unchanged in every subdivision[455]
from the largest masses to the smallest particles, and consequently
these characteristics must be properties of the particles. But the world of
heavenly luminaries appeared equally fanciful at man's first acquaintance
with it, so much so, that the astrologers imagined a connection between the
individualities of men and the conjunctions of planets. Thanks to the genius
of Lavoisier and of Dalton, man has been able, in the unseen world of chemical
combinations, to recognise laws of the same simple order as those
which Copernicus and Kepler proved to exist in the planetary universe. Man
discovered, and continues every hour to discover, what remains unchanged
in chemical evolution, and how changes take place in combinations of the
unchangeable. He has learned to predict, not only what possible combinations
may take place, but also the very existence of atoms of unknown elementary
substances, and has besides succeeded in making innumerable practical
applications of his knowledge to the great advantage of his race, and has
accomplished this notwithstanding that notions of sympathy and affinity
still preserve a strong vitality in science. At present we cannot apply
Newton's principles to chemistry, because the soil is only being now prepared.
The invisible world of chemical atoms is still waiting for the creator of chemical
mechanics. For him our age is collecting a mass of materials, the
inductions of well-digested facts, and many-sided inferences similar to those
which existed for Astronomy and Mechanics in the days of Newton. It is
well also to remember that Newton devoted much time to chemical experiments,
and while considering questions of celestial mechanics, persistently
kept in view the mutual action of those infinitely small worlds which are
concerned in chemical evolutions. For this reason, and also to maintain the
unity of laws, it seems to me that we must, in the first instance, seek to
harmonise the various phases of contemporary chemical theories with the
immortal principles of the Newtonian natural philosophy, and so hasten the
advent of true chemical mechanics. Let the above considerations serve as
my justification for the attempt which I propose to make to act as a champion
of the universality of the Newtonian principles, which I believe are competent
to embrace every phenomenon in the universe, from the rotation of
the fixed stars to the interchanges of chemical atoms.
In the first place I consider it indispensable to bear in mind that, up to
quite recent times, only a one-sided affinity has been recognised in chemical
reactions. Thus, for example, from the circumstance that red-hot iron decomposes
water with the evolution of hydrogen, it was concluded that oxygen
had a greater affinity for iron than for hydrogen. But hydrogen, in presence
of red-hot iron scale, appropriates its oxygen and forms water, whence an
exactly opposite conclusion may be formed.
During the last ten years a gradual, scarcely perceptible, but most
important change has taken place in the views, and consequently in the
researches, of chemists. They have sought everywhere, and have always
found, systems of conservation or dynamic equilibrium substantially similar
to those which natural philosophers have long since discovered in the visible
world, and in virtue of which the position of the heavenly bodies in the
universe is determined. There where one-sided affinities only were at first
detected, not only secondary or lateral ones have been found, but even those[456]
which are diametrically opposite; yet among these, dynamical equilibrium
establishes itself not by excluding one or other of the forces, but regulating
them all. So the chemist finds in the flame of the blast furnace, in the
formation of every salt, and, with especial clearness, in double salts and in
the crystallisation of solutions, not a fight ending in the victory of one side,
as used to be supposed, but the conjunction of forces; the peace of dynamic
equilibrium resulting from the action of many forces and affinities. Carbonaceous
matters, for example, burn at the expense of the oxygen of the
air, yielding a quantity of heat, and forming products of combustion, in
which it was thought that the affinities of the oxygen with the combustible
elements were satisfied. But it appeared that the heat of combustion was
competent to decompose these products, to dissociate the oxygen from the
combustible elements, and therefore to explain combustion fully it is necessary
to take into account the equilibrium between opposite reactions, between
those which evolve and those which absorb heat.
In the same way, in the case of the solution of common salt in water, it
is necessary to take into account, on the one hand, the formation of compound
particles generated by the combination of salt with water, and, on the other,
the disintegration or scattering of the new particles formed, as well as of
these originally contained. At present we find two currents of thought,
apparently antagonistic to each other, dominating the study of solutions:
according to the one, solution seems a mere act of building up or association;
according to the other, it is only dissociation or disintegration. The truth
lies, evidently, between these views; it lies, as I have endeavoured to prove
by my investigations into aqueous solutions, in the dynamic equilibrium of
particles tending to combine and also to fall asunder. The large majority of
chemical reactions which appeared to act victoriously along one line have
been proved capable of acting as victoriously even along an exactly opposite
line. Elements which utterly decline to combine directly may often be
formed into comparatively stable compounds by indirect means, as, for example,
in the case of chlorine and carbon; and consequently the sympathies
and antipathies which it was thought to transfer from human relations to
those of atoms should be laid aside until the mechanism of chemical relations
is explained. Let us remember, however, that chlorine, which does not
form with carbon the chloride of carbon, is strongly absorbed, or, as it were,
dissolved, by carbon, which leads us to suspect incipient chemical action even
in an external and purely surface contact, and involuntarily gives rise to
conceptions of that unity of the forces of nature which has been so energetically
insisted on by Sir William Grove and formulated in his famous
paradox. Grove noticed that platinum, when fused in the oxyhydrogen
flame, during which operation water is formed, when allowed to drop into
water decomposes the latter and produces the explosive oxyhydrogen mixture.
The explanation of this paradox, as of many others which arose during the
period of chemical renaissance, has led, in our time, to the promulgation by
Henri Sainte-Claire Deville of the conception of dissociation and of equilibrium,
and has recalled the teaching of Berthollet, which, notwithstanding its
brilliant confirmation by Heinrich Rose and Dr. Gladstone, had not, up to
that period, been included in received chemical views.
[457]
Chemical equilibrium in general, and dissociation in particular, are now
being so fully worked out in detail, and supplied in such various ways, that I
do not allude to them to develop, but only use them as examples by which
to indicate the correctness of a tendency to regard chemical combinations
from points of view differing from those expressed by the term hitherto appropriated
to define chemical forces, namely, ‘affinity.’ Chemical equilibria,
dissociation, the speed of chemical reactions, thermochemistry, spectroscopy,
and, more than all, the determination of the influence of masses and the
search for a connection between the properties and weights of atoms and
molecules—in one word, the vast mass of the most important chemical researches
of the present day—clearly indicate the near approach of the time
when chemical doctrines will submit fully and completely to the doctrine
which was first announced in the Principia of Newton.
In order that the application of these principles may bear fruit it is evidently
insufficient to assume that statical equilibrium reigns alone in chemical
systems or chemical molecules: it is necessary to grasp the conditions of
possible states of dynamical equilibria, and to apply to them kinetic principles.
Numerous considerations compel us to renounce the idea of statical
equilibrium in molecules, and the recent yet strongly-supported appeals to
dynamic principles constitute, in my opinion, the foundation of the modern
teaching relating to atomicity, or the valency of the elements, which usually
forms the basis of investigations into organic or carbon compounds.
This teaching has led to brilliant explanations of very many chemical
relations and to cases of isomerism, or the difference in the properties of
substances having the same composition. It has been so fruitful in its many
applications and in the foreshadowing of remote consequences, especially
respecting carbon compounds, that it is impossible to deny its claims to be
ranked as a great achievement of chemical science. Its practical application
to the synthesis of many substances of the most complicated composition
entering into the structure of organised bodies, and to the creation of an unlimited
number of carbon compounds, among which the colours derived from
coal tar stand prominently forward, surpass the synthetical powers of Nature
itself. Yet this teaching, as applied to the structure of carbon compounds,
is not on the face of it directly applicable to the investigation of other elements,
because in examining the first it is possible to assume that the atoms
of carbon have always a definite and equal number of affinities, whilst in the
combinations of other elements this is evidently inadmissible. Thus, for
example, an atom of carbon yields only one compound with four atoms of
hydrogen and one with four atoms of chlorine in the molecule, whilst the
atoms of chlorine and hydrogen unite only in the proportions of one to one.
Simplicity is here evident, and forms a point of departure from which it is
easy to move forward with firm and secure tread. Other elements are of a
different nature. Phosphorus unites with three and with five atoms of
chlorine, and consequently the simplicity and sharpness of the application of
structural conceptions are lost. Sulphur unites only with two atoms of
hydrogen, but with oxygen it enters into higher orders of combination. The
periodic relationship which exists among all the properties of the elements—such,
for example, as their ability to enter into various combinations—and[458]
their atomic weights, indicate that this variation in atomicity is subject to
one perfectly exact and general law, and it is only carbon and its near
analogues which constitute cases of permanently preserved atomicity. It is
impossible to recognise as constant and fundamental properties of atoms,
powers which, in substance, have proved to be variable. But by abandoning
the idea of permanence, and of the constant saturation of affinities—that is
to say, by acknowledging the possibility of free affinities—many retain a
comprehension of the atomicity of the elements ‘under given conditions;’
and on this frail foundation they build up structures composed of chemical
molecules, evidently only because the conception of manifold affinities gives,
at once, a simple statical method of estimating the composition of the most
complicated molecules.
I shall enter neither into details, nor into the various consequences following
from these views, nor into the disputes which have sprung up respecting
them (and relating especially to the number of isomerides possible on the
assumption of free affinities), because the foundation or origin of theories of
this nature suffers from the radical defect of being in opposition to dynamics.
The molecule, as even Laurent expressed himself, is represented as an architectural
structure, the style of which is determined by the fundamental
arrangement of a few atoms, whilst the decorative details, which are capable
of being varied by the same forces, are formed by the elements entering into
the combination. It is on this account that the term ‘structural’ is so appropriate
to the contemporary views of the above order, and that the ‘structuralists’
seek to justify the tetrahedric, plane, or prismatic disposition of
the atoms of carbon in benzene. It is evident that the consideration relates
to the statical position of atoms and molecules and not to their kinetic relations.
The atoms of the structural type are like the lifeless pieces on a chess
board: they are endowed but with the voices of living beings, and are not
those living beings themselves; acting, indeed, according to laws, yet each
possessed of a store of energy which, in the present state of our knowledge,
must be taken into account.
In the days of Haüy, crystals were considered in the same statical and
structural light, but modern crystallographers, having become more thoroughly
acquainted with their physical properties and their actual formation,
have abandoned the earlier views, and have made their doctrines dependent
on dynamics.
The immediate object of this lecture is to show that, starting with
Newton's third law of motion, it is possible to preserve to chemistry all the
advantages arising from structural teaching, without being obliged to build
up molecules in solid and motionless figures, or to ascribe to atoms definite
limited valencies, directions of cohesion, or affinities. The wide extent of
the subject obliges me to treat only a small portion of it, namely of substitutions,
without specially considering combinations and decompositions, and
even then limiting myself to the simplest examples, which, however, will
throw open prospects embracing all the natural complexity of chemical relations.
For this reason, if it should prove possible to form groups similar, for
example, to H4 or CH6 as the remnants of molecules CH4 or C2H7 we shall
not pause to consider them, because, as far as we know, they fall asunder into[459]
two parts, H2 + H2 or CH4 + H2, as soon as they are even temporarily formed,
and are incapable of separate existence, and therefore can take no part in
the elementary act of substitution. With respect to the simplest molecules
which we shall select—that is to say, those of which the parts have no separate
existence, and therefore cannot appear in substitutions—we shall consider
them according to the periodic law, arranging them in direct dependence
on the atomic weight of the elements.
Thus, for example, the molecules of the simplest hydrogen compounds—
| HF |
H2O |
H3N |
H4C |
| hydrofluoric acid |
water |
ammonia |
Hmethane |
correspond with elements the atomic weights of which decrease consecutively
F = 19, O = 16, N = 14, C = 12.
Neither the arithmetical order (1, 2, 3, 4 atoms of hydrogen) nor the total
information we possess respecting the elements will permit us to interpolate
into this typical series one more additional element; and therefore we have
here, for hydrogen compounds, a natural base on which are built up those
simple chemical combinations which we take as typical. But even they are
competent to unite with each other, as we see, for instance, in the property
which hydrofluoric acid has of forming a hydrate—that is, of combining with
water; and a similar attribute of ammonia, resulting in the formation of a
caustic alkali, NH3,H2O, or NH4OH.
Having made these indispensable preliminary observations, I may now
attack the problem itself and attempt to explain the so-called structure or
rather construction, of molecules—that is to say, their constitution and transformations—without
having recourse to the teaching of ‘structuralists,’ but on
Newton's dynamical principles.
Of Newton's three laws of motion, only the third can be applied directly
to chemical molecules when regarded as systems of atoms among which it
must be supposed that there exist common influences or forces, and resulting
compounded relative motions. Chemical reactions of every kind are undoubtedly
accomplished by changes in these internal movements, respecting
the nature of which nothing is known at present, but the existence of which
the mass of evidence collected in modern times forces us to acknowledge as
forming part of the common motion of the universe, and as a fact further
established by the circumstance that chemical reactions are always characterised
by changes of volume or the relations between the atoms or the
molecules. Newton's third law, which is applicable to every system, declares
that, ‘action is also associated with reaction, and is equal to it.’ The
brevity of conciseness of this axiom was, however, qualified by Newton in
a more expanded statement, ‘the action of bodies one upon another are
always equal, and in opposite directions.’ This simple fact constitutes the
point of departure for explaining dynamic equilibrium—that is to say, systems
of conservancy. It is capable of satisfying even the dualists, and of explaining,
without additional assumptions, the preservation of those chemical types
which Dumas, Laurent, and Gerhardt created unit types, and those views of
atomic combinations which the structuralists express by atomicity or the[460]
valency of the elements, and, in connection with them, the various numbers
of affinities. In reality, if a system of atoms or a molecule be given, then in
it, according to the third law of Newton, each portion of atoms acts on the
remaining portion in the same manner, and with the same force as the
second set of atoms acts on the first. We infer directly from this consideration
that both sets of atoms, forming a molecule, are not only equivalent with
regard to themselves, as they must be according to Dalton's law, but also that
they may, if united, replace each other. Let there be a molecule containing
atoms A B C, it is clear that, according to Newton's law, the action of A on
B C must be equal to the action of B C on A, and if the first action is directed
on B C, then the second must be directed on A, and consequently then, where
A can exist in dynamic equilibrium, B C may take its place and act in a like
manner. In the same way the action of C is equal to the action of A B. In
one word every two sets of atoms forming a molecule are equivalent to each
other, and may take each other's place in other molecules, or, having the
power of balancing each other, the atoms or their complements are endowed
with the power of replacing each other. Let us call this consequence of an
evident axiom ‘the principle of substitution,’ and let us apply it to those
typical forms of hydrogen compounds which we have already discussed, and
which, on account of their simplicity, and regularity, have served as starting-points
of chemical argument long before the appearance of the doctrine of
structure.
In the type of hydrofluoric acid, HF, or in systems of double stars, are
included a multitude of the simplest molecules. It will be sufficient for our
purpose to recall a few: for example, the molecules of chlorine, Cl2, and of
hydrogen, H2, and hydrochloric acid, HCl, which is familiar to all in aqueous
solution as spirits of salt, and which has many points of resemblance with
HF, HBr, HI. In these cases division into two parts can only be made in
one way, and therefore the principle of substitution renders it probable that
exchanges between the chlorine and the hydrogen can take place, if they are
competent to unite with each other. There was a time when no chemist
would even admit the idea of any such action; it was then thought that the
power of combination indicated a polar difference of the molecules in combination,
and this thought set aside all idea of the substitution of one component
element by another.
Thanks to the observations and experiments of Dumas and Laurent fifty
years ago, such fallacies were dispelled, and in this manner the principle
of substitution was exhibited. Chlorine and bromine acting on many
hydrogen compounds, occupy immediately the place of their hydrogen, and
the displaced hydrogen, with another atom of chlorine or bromine, forms
hydrochloric acid or bromide of hydrogen. This takes place in all typical
hydrogen compounds. Thus chlorine acts on this principle on gaseous
hydrogen—reaction, under the influence of light, resulting in the formation
of hydrochloric acid. Chlorine acting on the alkalis, constituted similarly to
water, and even on water itself—only, however, under the influence of light
and only partially because of the instability of HClO—forms by this principle
bleaching salts, which are the same as the alkalis, but with their hydrogen
replaced by chlorine. In ammonia and in methane, chlorine can also replace[461]
the hydrogen. From ammonia is formed in this manner the so-called
chloride of nitrogen, NCl3, which decomposes very readily with violent explosion
on account of the evolved gases, and falls asunder as chlorine and
nitrogen. Out of marsh gas, or methane, CH4, may be obtained consecutively,
by this method, every possible substitution, of which chloroform,
CHCl3, is the best known, and carbon tetrachloride, CCl4, the most instructive.
But by virtue of the fact that chlorine and bromine act, in the manner
shown, on the simplest typical hydrogen compounds, their action on the
more complicated ones may be assumed to be the same. This can be easily
demonstrated. The hydrogen of benzene, C6H6, reacts feebly under the influence
of light on liquid bromine, but Gustavson has shown that the addition
of the smallest quantity of metallic aluminium causes energetic action and
the evolution of large volumes of hydrogen bromide.
If we pass on to the second typical hydrogen compound—that is to say,
water—its molecule, HOH, may be split up in two ways: either into an atom
of hydrogen and a semi-molecule of hydrogen peroxide, HO, or into oxygen,
O, and two atoms of hydrogen, H; and therefore, according to the principle
of substitution, it is evident that one atom of hydrogen can exchange
with hydrogen oxide, HO, and two atoms of hydrogen, H, with one atom of
oxygen, O.
Both these forms of substitution will constitute methods of oxidation—that
is to say, of the entrance of oxygen into the compound—a reaction
which is so common in nature as well as in the arts, taking place at the
expense of the oxygen of the air or by the aid of various oxidising substances
or bodies which part easily with their oxygen. There is no occasion
to reckon up the unlimited number of cases of such oxidising reactions. It
is sufficient to state that in the first of these oxygen is directly transferred,
and the position, the chemical function, which hydrogen originally occupied,
is, after the substitution, occupied by the hydroxyl. Thus ammonia, NH3,
yields hydroxylamine, NH2(OH), a substance which retains many of the
properties of ammonia.
Methane and a number of other hydrocarbons yield, by substitution of
the hydrogen by its oxide, methyl alcohol, CH3(OH), and other alcohols. The
substitution of one atom of oxygen for two atoms of hydrogen is equally
common with hydrogen compounds. By this means alcoholic liquids containing
ethyl alcohol, or spirits of wine, C2H5(OH), are oxidised until they
become vinegar, or acetic acid, C2H3O(OH). In the same way caustic
ammonia, or the combination of ammonia with water, NH3,H2O, or NH4(OH),
which contains a great deal of hydrogen, by oxidation exchanges four atoms
of hydrogen for two atoms of oxygen, and becomes converted into nitric acid,
NO2(OH). This process of conversion of ammonium salts into saltpetre goes
on in the fields every summer, and with especial rapidity in tropical countries.
The method by which this is accomplished, though complex, though involving
the agency of all-permeating micro-organisms, is, in substance, the same as
that by which alcohol is converted into acetic acid, or glycol, C2H4(OH)2, into
oxalic acid, if we view the process of oxidation in the light of the Newtonian
principles.
But while speaking of the application of the principle of substitution to[462]
water, we need not multiply instances, but must turn our attention to two
special circumstances which are closely connected with the very mechanism
of substitutions.
In the first place, the replacement of two atoms of hydrogen by one atom
of oxygen may take place in two ways, because the hydrogen molecule is
composed of two atoms, and therefore, under the influence of oxygen, the
molecule forming water may separate before the oxygen has time to take its
place. It is for this reason that we find, during the conversion of alcohol
into acetic acid, that there is an interval during which is formed aldehyde,
C2H4O, which, as its very name implies, is ‘alcohol dehydrogenatum,’ or
alcohol deprived of hydrogen. Hence aldehyde combined with hydrogen
yields alcohol; and united to oxygen, acetic acid.
For the same reason there should be, and there actually are, intermediate
products between ammonia and nitric acid, NO2(HO), containing either less
hydrogen than ammonia, less oxygen than nitric acid, or less water than
caustic ammonia. Accordingly we find, among the products of the deoxidation
of nitric acid and the oxidation of ammonia, not only hydroxylamine,
but also nitrous oxide, nitrous and nitric anhydrides. Thus, the production
of nitrous acid results from the removal of two atoms of hydrogen from
caustic ammonia and the substitution of the oxygen for the hydrogen,
NO(OH); or by the substitution, in ammonia, of three atoms of hydrogen by
hydroxyl, N(OH)3, and by the removal of water: N(OH)3 - H2O = NO(OH).
The peculiarities and properties of nitrous acid—as, for instance, its action on
ammonia and its conversion, by oxidation, into nitric acid—are thus clearly
revealed.
On the other hand, in speaking of the principle of substitution as applied
to water, it is necessary to observe that hydrogen and hydroxyl, H and OH,
are not only competent to unite, but also to form combinations with themselves,
and thus become H2 and H2O2; and such are hydrogen and the
peroxide thereof. In general, if a molecule A B exists, then molecules A A
and B B can exist also. A direct reaction of this kind does not, however,
take place in water, therefore undoubtedly, at the moment of formation,
hydrogen reacts on hydrogen peroxide, as we can show at once by
experiment; and further because hydrogen peroxide, H2O2, exhibits a
structure containing a molecule of hydrogen, H2, and one of oxygen, O2,
either of which is capable of separate existence. The fact, however, may
now be taken as thoroughly established, that, at the moment of combustion
of hydrogen or of the hydrogen compounds, hydrogen peroxide is always
formed, and not only so, but in all probability its formation invariably precedes
the formation of water. This was to be expected as a consequence of
the law of Avogadro and Gerhardt, which leads us to expect this sequence
in the case of equal interactions of volumes of vapours and gases; and in
hydrogen peroxide we actually have such equal volumes of the elementary
gases.
The instability of hydrogen peroxide—that is to say, the ease with
which it decomposes into water and oxygen, even at the mere contact of
porous substances—accounts for the circumstance that it does not form a permanent
product of combustion, and is not produced during the decomposition[463]
of water. I may mention this additional consideration that, with respect
to hydrogen peroxide, we may look for its effecting still further substitutions
of hydrogen by means of which we may expect to obtain still more
highly oxidised water compounds, such as H2O3 and H2O4. These Schönbein
and Bunsen have long been seeking, and Berthelot is investigating them
at present. It is probable, however, that the reaction will stop at the
last compound, because we find that, in a number of cases, the addition of
four atoms of oxygen seems to form a limit. Thus, OsO4, KClO4, KMnO4,
K2SO4, Na3PO4, and such like, represent the highest grades of oxidation.[1]
As for the last forty years, from the times of Berzelius, Dumas, Liebig,
Gerhardt, Williamson, Frankland, Kolbe, Kekulé, and Butleroff, most theoretical
generalisations have centred round organic or carbon compounds,
we will, for the sake of brevity, leave out the discussion of ammonia derivatives,
notwithstanding their simplicity with respect to the doctrine of substitutions;
we will dwell more especially on its application to carbon compounds,
starting from methane, CH4, as the simplest of the hydrocarbons, containing
in its molecule one atom of carbon. According to the principles enumerated
we may derive from CH4 every combination of the form CH3X, CH2X2,
CHX3, and CX4, in which X is an element, or radicle, equivalent to hydrogen—that
is to say, competent to take its place or to combine with it. Such are
the chlorine substitutes already mentioned, such is wood-spirit, CH3(OH), in
which X is represented by the residue of water, and such are numerous other
carbon derivatives. If we continue, with the aid of hydroxyl, further substitutions
of the hydrogen of methane we shall obtain successively CH2(OH)2,
CH(OH)3, and C(OH)4. But if, in proceeding thus, we bear in mind that
CH2(OH)2 contains two hydroxyls in the same form as hydrogen peroxide,
H2O2 or (OH)2, contains them—and moreover not only in one molecule, but
together, attached to one and the same atom of carbon—so here we must
look for the same decomposition as that which we find in hydrogen peroxide,
and accompanied also by the formation of water as an independently
existing molecule; therefore CH2(OH)2 should yield, as it actually does, immediately
water and the oxide of methylene, CH2O, which is methane with[464]
oxygen substituted for two atoms of hydrogen. Exactly in the same manner
out of CH(OH)3 are formed water and formic acid, CHO(OH), and out of
C(OH)4 is produced water and carbonic acid, or directly carbonic anhydride,
CO2, which will therefore be nothing else than methane with the double replacement
of pairs of hydrogen by oxygen. As nothing leads to the supposition
that the four atoms of hydrogen in methane differ one from the other,
so it does not matter by what means we obtain any one of the combinations
indicated—they will be identical; that is to say, there will be no case of
actual isomerism, although there may easily be such cases of isomerism as
have been distinguished by the term metamerism.
Formic acid, for example, has two atoms of hydrogen, one attached to the
carbon left from the methane, and the other attached to the oxygen which
has entered in the form of hydroxyl, and if one of them be replaced by some
substance X it is evident that we shall obtain substances of the same composition,
but of different construction, or of different orders of movement among
the molecules, and therefore endowed with other properties and reactions. If
X be methyl, CH4—that is to say, a group capable of replacing hydrogen
because it is actually contained with hydrogen in methane itself—then by
substituting this group for the original hydrogen we obtain acetic acid,
CCH3O(OH), out of formic, and by substitution of the hydrogen in its oxide or
hydroxyl we obtain methyl formate, CHO(OCH3). These substances differ so
much from each other physically and chemically that at first sight it is hardly
possible to admit that they contain the same atoms in identically the same
proportions. Acetic acid, for example, boils at a higher temperature than
water, and has a higher specific gravity than it, whilst its metameride,
methyl formate, is lighter than water, and boils at 30°—that is to say, it
evaporates very easily.
Let us now turn to carbon compounds containing two atoms of carbon to
the molecule, as in acetic acid, and proceed to evolve them from methane by
the principle of substitution. This principle declares at once that methane
can only be split up in the four following ways:—
1. Into a group CH3 equivalent with H. Let us call changes of this
nature methylation.
2. Into a group CH2 and H2. We will call this order of substitutions
methylenation.
3. Into CH and H3, which commutations we will call acetylenation.
4. Into C and H4, which may be called carbonation.
It is evident that hydrocarbon compounds containing two atoms of carbon
can only proceed from methane, CH4, which contains four atoms of hydrogen
by the first three methods of substitution; carbonation would yield free carbon
if it could take place directly, and if the molecule of free carbon—which is in
reality very complex, that is to say strongly polyatomic, as I have long since
been proving by various means—could contain only C2 like the molecules
O2, H2, N2, and so on.
By methylation we should evidently obtain from marsh gas, ethane,
CH3CH3 = C2H6.
By methylenation—that is, by substituting group CH2 for H2—methane
forms ethylene, CH2CH2 = C2H4.
[465]
By acetylenation—that is, by substituting three atoms of hydrogen, H3, in
methane—by the remnant CH, we get acetylene, CHCH = C2H2.
If we have applied the principles of Newton correctly, there should not be
any other hydrocarbons containing two atoms of carbon in the molecule.
All these combinations have long been known, and in each of them we can
not only produce those substitutions of which an example has been given in
the case of methane, but also all the phases of other substitutions, as we shall
find from a few more instances, by the aid of which I trust that I shall be
able to show the great complexity of those derivatives which, on the principle
of substitution, can be obtained from each hydrocarbon. Let us content ourselves
with the case of ethane, CH3CH3, and the substitution of the hydrogen
by hydroxyl. The following are the possible changes:—
1. CH3CH2(OH): this is nothing more than spirit of wine, or ethyl
alcohol, C2H5(OH) or C2H6O.
2. CH2(OH)CH2(OH): this is the glycol of Würtz, which has shed so
much light on the history of alcohol. Its isomeride may be CH3CH(OH)2,
but as we have seen in the case of CH(OH)2, it decomposes, giving off water,
and forming aldehyde, CH3CHO, a substance capable of yielding alcohol by
uniting with hydrogen, and of yielding acetic acid by uniting with oxygen.
If glycol, CH2(OH)CH2(OH), loses its water, it may be seen at once that
it will not now yield aldehyde, CH3CHO, but its isomeride, CH2CH2/O, the
oxide of ethylene. I have here indicated in a special manner the oxygen
which has taken the place of two atoms of the hydrogen of ethane taken
from different atoms of the carbon.
3. CH3C(OH)3 decomposed as CH(OH)3, forming water and acetic acid,
CH3CO(OH). It is evident that this acid is nothing else than formic acid,
CHO(OH), with its hydrogen replaced by methyl. Without examining
further the vast number of possible derivatives, I will direct your attention
to the circumstance that in dissolving acetic acid in water we obtain the
maximum contraction and the greatest viscosity when to the molecule
CH3CO(OH) is added a molecule of water, which is the proportion which
would form the hydrate CH3C(OH)3. It is probable that the doubling of
the molecule of acetic acid at temperatures approaching its boiling-point
has some connection with this power of uniting with one molecule of
water.
4. CH2(OH)C(OH)3 is evidently an alcoholic acid, and indeed this compound,
after losing water, answers to glycolic acid, CH2(OH)CO(OH).
Without investigating all the possible isomerides, we will note only that the
hydrate CH(OH)2CH(OH)2 has the same composition as CH2(OH)C(OH)3,
and although corresponding to glycol, and being a symmetrical substance, it
becomes, on parting with its water, the aldehyde of oxalic acid, or the glyoxal
of Debus, CHOCHO.
5. CH(OH)2C(OH3), from the tendency of all the preceding, corresponds
with glyoxylic acid, an aldehyde acid, CHOCO(OH), because the group
CO(OH), or carboxyl, enters into the compositions of organic acids, and the
group CHO defines the aldehyde function.
6. C(OH)3C(OH)3 through the loss of 2H2O yields the bibasic oxalic acid[466]
CO(OH)CO(OH), which generally crystallises with 2H2O, following thus the
normal type of hydration characteristic of ethane.[2]
Thus, by applying the principle of substitution, we can, in the simplest
manner, derive not only every kind of hydrocarbon compound, such as the
alcohols, the aldehyde-alcohols, aldehydes, alcohol-acids, and the acids, but
also combinations analogous to hydrated crystals which usually are disregarded.
But even those unsaturated substances, of which ethylene, CH2CH2, and
acetylene, CHCH, are types, may be evolved with equal simplicity. With
respect to the phenomena of isomerism, there are many possibilities among
the hydrocarbon compounds containing two atoms of carbon, and without
going into details it will be sufficient to indicate that the following formulæ,
though not identical, will be isomeric substantially among themselves:—CH3CHX2
and CH2XCH2X, although both contain C2H4X2; or CH2CX2 and
CHXCHX, although both contain C2H2X2, if by X we indicate chlorine or
generally an element capable of replacing one atom of hydrogen, or capable
of uniting with it. To isomerism of this kind belongs the case of aldehyde
and the oxide of ethylene, to which we have already referred, because both
have the composition C2H4O.
What I have said appears to me sufficient to show that the principle of
substitution adequately explains the composition, the isomerism, and all the
diversity of combination of the hydrocarbons, and I shall limit the further
development of these views to preparing a complete list of every possible
hydrocarbon compound containing three atoms of carbon in the molecule.
There are eight in all, of which only five are known at present.[3]
Among those possible for C3H6 there should be two isomerides, propylene
and trimethylene, and they are both already known. For C3H4 there should
be three isomerides: allylene and allene are known, but the third has not
yet been discovered; and for C3H2 there should be two isomerides, though
neither of them is known as yet. Their composition and structure are easily[467]
deduced from ethane, ethylene, and acetylene, by methylation, by methylenation,
by acetylenation and by carbonation.
1. C3H8 = CH3CH2CH3 out of CH3CH3 by methylation. This hydrocarbon
is named propane.
2. C3H6 = CH3CHCH2 out of CH3CH3 by methylenation. This substance
is propylene.
3. C3H6 = CH2CH2CH2 out of CH3CH3 by methylenation. This substance
is trimethylene.
4. C3H4 = CH3CCH out of CH3CH3 by acetylenation or from CHCH by
methylation. This hydrocarbon is named allylene.
5. C3H4 =
CHCH
CH2
out of CH3CH3 by acetylenation, or from CH2CH2 by
methylenation, because
CH2CH
CH
=
CHCH
CH2
. This body is as yet unknown.
6. C3H4 = CH2CCH2 out of CH2CH2 by methylenation. This hydrocarbon
is named allene, or iso-allylene.
7. C3H2 =
CHCH
C
out of CH3CH3 by symmetrical carbonation, or out
of CH2CH2 by acetylenation. This compound is unknown.
8. C3H2 =
CC
CH2
out of CH3CH3 by carbonation, or out of CHCH by
methylenation. This compound is unknown.
If we bear in mind that for each hydrocarbon serving as a type in the
above tables there are a number of corresponding derivatives, and that every
compound obtained may, by further methylation, methylenation, acetylenation,
and carbonation, produce new hydrocarbons, and these may be followed
by a numerous suite of derivatives and an immense number of isomeric
substances, it is possible to understand the limitless number of carbon compounds,
although they all have the one substance, methane, for their origin.
The number of substances is so enormous that it is no longer a question of
enlarging the possibilities of discovery, but rather of finding some means of
testing them analogous to the well-known two which for a long time have
served as gauges for all carbon compounds.
I refer to the law of even numbers and to that of limits, the first enunciated
by Gerhardt some forty years ago, with respect to hydrocarbons, namely,
that their molecules always contain an even number of atoms of hydrogen.
But by the method which I have used of deriving all the hydrocarbons from
methane, CH4, this law may be deduced as a direct consequence of the
principle of substitutions. Accordingly, in methylation, CH3 takes the place
of H, and therefore CH2 is added. In methylenation the number of atoms of
hydrogen remains unchanged, and at each acetylenation it is reduced by two,
and in carbonation by four, atoms—that is to say, an even number of atoms
of hydrogen is always added or removed. And because the fundamental
hydrocarbon, methane, CH4, contains an even number of atoms of hydrogen,
all its derivative hydrocarbons will also contain even numbers of hydrogen,
and this constitutes the law of even numbers.
The principle of substitutions explains with equal simplicity the conception
of the limiting compositions of hydrocarbons CnH2n+2, which I derived, in[468]
1861,[4] in an empirical manner from accumulated materials available at that
time, and on the basis of the limits to combinations worked out by Dr. Frankland
for other elements.
Of all the various substitutions the highest proportion of hydrogen is
yielded by methylation, because in that operation alone does the quantity of
hydrogen increase; hence, taking methane as a point of departure, if we
imagine methylation effected (n - 1) times we obtain hydrocarbon compounds
containing the highest quantities of hydrogen. It is evident that they will
contain CH4 + (n - 1)CH2, or CnH2n+2, because methylation leads to the addition
of CH2 to the compound.
It will thus be seen that by the principle of substitution—that is to say,
by the third law of Newton—we are able to deduce, in the simplest manner,
not only the individual composition, the isomerism, and relations of substances,
but also the general laws which govern their most complex combinations
without having recourse either to statical constructions, to the definition
of atomicities, to the exclusion of free affinities, or to the recognition of those
single, double or treble bonds which are so indispensable to structuralists in the
explanation of the composition and construction of hydrocarbon compounds.
And yet, by the application of the dynamical principles of Newton, we can
attain to that chief and fundamental object, the comprehension of isomerism
in hydrocarbon compounds, and the forecasting of the existence of combinations
as yet unknown, by which the edifice raised by structural teaching is
strengthened and supported. Besides—and I count this for a circumstance
of special importance—the process which I advocate will make no difference
in those special cases which have been already so well worked out, such as,
for example, the isomerism of the hydrocarbons and alcohols, even to the
extent of not interfering with the nomenclature which has been adopted, and
the structural system will retain all the glory of having worked up, in a
thoroughly scientific manner, the store of information which Gerhardt had
accumulated about the middle of the fifties, and the still higher glory of
establishing the rational synthesis of organic substances. Nothing will be
lost to the structural doctrine except its statical origin; and as soon as it
will embrace the dynamic principles of Newton, and suffer itself to be guided
by them, I believe that we shall attain for chemistry that unity of principle
which is now wanting. Many an adept will be attracted to that brilliant and
fascinating enterprise, the penetration into the unseen world of the kinetic
relations of atoms, to the study of which the last twenty-five years have contributed
so much labour and such high inventive faculties.
D'Alembert found in mechanics that if inertia be taken to represent force,
dynamic equations may be applied to statical questions, which are thereby
rendered more simple and more easily understood.
The structural doctrine in chemistry has unconsciously followed the same
course, and therefore its terms are easily adopted; they may retain their
present forms provided that a truly dynamical—that is to say, Newtonian—meaning
be ascribed to them.
Before finishing my task and demonstrating the possibility of adapting[469]
structural doctrines to the dynamics of Newton, I consider it indispensable
to touch on one question which naturally arises, and which I have heard
discussed more than once. If bromine, the atom of which is eighty times
heavier than that of hydrogen, takes the place of hydrogen, it would seem
that the whole system of dynamic equilibrium must be destroyed.
Without entering into the minute analysis of this question, I think it
will be sufficient to examine it by the light of two well-known phenomena,
one of which will be found in the department of chemistry and the other in
that of celestial mechanics, and both will serve to demonstrate the existence
of that unity in the plan of creation which is a consequence of the Newtonian
doctrines. Experiments demonstrate that when a heavy element is substituted
for a light one in a chemical compound—for example, for magnesium,
in the oxide of that metal, an atom of mercury, which is 8⅓ times heavier—the
chief chemical characteristics or properties are generally, though not
always, preserved.
The substitution of silver for hydrogen, than which it is 108 times heavier,
does not affect all the properties of the substance, though it does some.
Therefore chemical substitutions of this kind—the substitution of light for
heavy atoms—need not necessarily entail changes in the original equilibrium;
and this point is still further elucidated by the consideration that the periodic
law indicates the degree of influence of an increment of weight in the atom
as affecting the possible equilibria, and also what degree of increase in the
weight of the atoms reproduces some, though not all, of the properties of the
substance.
This tendency to repetition—these periods—may be likened to those
annual or diurnal periods with which we are so familiar on the earth. Days
and years follow each other, but, as they do so, many things change; and in
like manner chemical evolutions, changes in the masses of the elements,
permit of much remaining undisturbed, though many properties undergo
alteration. The system is maintained according to the laws of conservation
in nature, but the motions are altered in consequence of the change of parts.
Next, let us take an astronomical case—such, for example, as the earth and
the moon—and let us imagine that the mass of the latter is constantly
increasing. The question is, what will then occur? The path of the moon
in space is a wave-line similar to that which geometricians have named epicycloidal,
or the locus of a point in a circle rolling round another circle. But
in consequence of the influence of the moon it is evident that the path of the
earth itself cannot be a geometric ellipse, even supposing the sun to be immovably
fixed; it must be an epicycloidal curve, though not very far removed
from the true ellipse—that is to say, it will be impressed with but faint undulations.
It is only the common centre of gravity of the earth and the
moon which describes a true ellipse round the sun. If the moon were to
increase, the relative undulations of the earth's path would increase in amplitude,
those of the moon would also change, and when the mass of the moon
had increased to an equality with that of the earth, the path would consist of
epicycloidal curves crossing each other, and having opposite phases. But a
similar relation exists between the sun and the earth, because the former is
also moving in space. We may apply these views to the world of atoms, and[470]
suppose that in their movements, when heavy ones take the place of those
that are lighter, similar changes take place, provided that the system or the
molecule is preserved throughout the change.
It seems probable that in the heavenly systems, during incalculable
astronomical periods, changes have taken place and are still going on similar
to those which pass rapidly before our eyes during the chemical reaction of
molecules, and the progress of molecular mechanics may—we hope will—in
course of time permit us to explain those changes in the stellar world which
have more than once been noticed by astronomers, and which are now so
carefully studied. A coming Newton will discover the laws of these changes.
Those laws, when applied to chemistry, may exhibit peculiarities, but these
will certainly be mere variations on the grand harmonious theme which
reigns in nature. The discovery of the laws which produce this harmony in
chemical evolution will only be possible, it seems to me, under the banner
of Newtonian dynamics, which has so long waved over the domains of
mechanics, astronomy, and physics. In calling chemists to take their stand
under its peaceful and catholic shadow I imagine that I am aiding in establishing
that scientific union which the managers of the Royal Institution
wish to effect, who have shown their desire to do so by the flattering invitation
which has given me—a Russian—the opportunity of laying before the
countrymen of Newton an attempt to apply to chemistry one of his immortal
principles.
[471]
APPENDIX II
THE PERIODIC LAW OF THE CHEMICAL ELEMENTS
By PROFESSOR MENDELÉEFF
FARADAY LECTURE DELIVERED BEFORE THE FELLOWS OF
THE CHEMICAL SOCIETY IN THE THEATRE OF THE ROYAL INSTITUTION, ON TUESDAY, JUNE 4, 1889
The high honour bestowed by the Chemical Society in inviting me to pay a
tribute to the world-famed name of Faraday by delivering this lecture has
induced me to take for its subject the Periodic Law of the Elements—this
being a generalisation in chemistry which has of late attracted much
attention.
While science is pursuing a steady onward movement, it is convenient
from time to time to cast a glance back on the route already traversed, and
especially to consider the new conceptions which aim at discovering the
general meaning of the stock of facts accumulated from day to day in our
laboratories. Owing to the possession of laboratories, modern science now
bears a new character, quite unknown, not only to antiquity, but even to the
preceding century. Bacon's and Descartes' idea of submitting the mechanism
of science simultaneously to experiment and reasoning has been fully realised
in the case of chemistry, it having become not only possible but always
customary to experiment. Under the all-penetrating control of experiment,
a new theory, even if crude, is quickly strengthened, provided it be founded
on a sufficient basis; the asperities are removed, it is amended by degrees,
and soon loses the phantom light of a shadowy form or of one founded on
mere prejudice; it is able to lead to logical conclusions, and to submit to experimental
proof. Willingly or not, in science we all must submit not to what
seems to us attractive from one point of view or from another, but to what
represents an agreement between theory and experiment; in other words, to
demonstrated generalisation and to the approved experiment. Is it long
since many refused to accept the generalisations involved in the law of Avogadro
and Ampère, so widely extended by Gerhardt? We still may hear the
voices of its opponents; they enjoy perfect freedom, but vainly will their
voices rise so long as they do not use the language of demonstrated facts[472]
The striking observations with the spectroscope which have permitted us to
analyse the chemical constitution of distant worlds, seemed, at first, applicable
to the task of determining the nature of the atoms themselves; but the
working out of the idea in the laboratory soon demonstrated that the characters
of spectra are determined, not directly by the atoms, but by the molecules
into which the atoms are packed; and so it became evident that more
verified facts must be collected before it will be possible to formulate new
generalisations capable of taking their place beside those ordinary ones based
upon the conception of simple substances and atoms. But as the shade of the
leaves and roots of living plants, together with the relics of a decayed vegetation,
favour the growth of the seedling and serve to promote its luxurious
development, in like manner sound generalisations—together with the relics
of those which have proved to be untenable—promote scientific productivity,
and ensure the luxurious growth of science under the influence of rays emanating
from the centres of scientific energy. Such centres are scientific
associations and societies. Before one of the oldest and most powerful of
these I am about to take the liberty of passing in review the twenty years' life
of a generalisation which is known under the name of the Periodic Law. It
was in March 1869 that I ventured to lay before the then youthful Russian
Chemical Society the ideas upon the same subject which I had expressed in
my just written ‘Principles of Chemistry.’
Without entering into details, I will give the conclusions I then arrived
at in the very words I used:—
‘1. The elements, if arranged according to their atomic weights, exhibit
an evident periodicity of properties.
‘2. Elements which are similar as regards their chemical properties have
atomic weights which are either of nearly the same value (e.g. platinum,
iridium, osmium) or which increase regularly (e.g. potassium, rubidium,
cæsium).
‘3. The arrangement of the elements, or of groups of elements, in the
order of their atomic weights, corresponds to their so-called valencies as well
as, to some extent, to their distinctive chemical properties—as is apparent,
among other series, in that of lithium, beryllium, barium, carbon, nitrogen,
oxygen, and iron.
‘4. The elements which are the most widely diffused have small atomic
weights.
‘5. The magnitude of the atomic weight determines the character of the
element, just as the magnitude of the molecule determines the character of
a compound.
‘6. We must expect the discovery of many yet unknown elements—for
example, elements analogous to aluminium and silicon, whose atomic weight
would be between 65 and 75.
‘7. The atomic weight of an element may sometimes be amended by a
knowledge of those of the contiguous elements. Thus, the atomic weight of
tellurium must lie between 123 and 126, and cannot be 128.
‘8. Certain characteristic properties of the elements can be foretold from
their atomic weights.
[473]
‘The aim of this communication will be fully attained if I succeed in
drawing the attention of investigators to those relations which exist between
the atomic weights of dissimilar elements, which, so far as I know, have
hitherto been almost completely neglected. I believe that the solution of
some of the most important problems of our science lies in researches of this
kind.’
To-day, twenty years after the above conclusions were formulated, they
may still be considered as expressing the essence of the now well-known
periodic law.
Reverting to the epoch terminating with the sixties, it is proper to indicate
three series of data without the knowledge of which the periodic law
could not have been discovered, and which rendered its appearance natural
and intelligible.
In the first place, it was at that time that the numerical value of atomic
weights became definitely known. Ten years earlier such knowledge did not
exist, as may be gathered from the fact that in 1860 chemists from all parts
of the world met at Karlsruhe in order to come to some agreement, if not
with respect to views relating to atoms, at any rate as regards their definite
representation. Many of those present probably remember how vain were
the hopes of coming to an understanding, and how much ground was gained
at that Congress by the followers of the unitary theory so brilliantly represented
by Cannizzaro. I vividly remember the impression produced by his
speeches, which admitted of no compromise, and seemed to advocate truth
itself, based on the conceptions of Avogadro, Gerhardt, and Regnault, which
at that time were far from being generally recognised. And though no
understanding could be arrived at, yet the objects of the meeting were attained,
for the ideas of Cannizzaro proved, after a few years, to be the only ones
which could stand criticism, and which represented an atom as—‘the
smallest portion of an element which enters into a molecule of its compound.’
Only such real atomic weights—not conventional ones—could afford a basis
for generalisation. It is sufficient, by way of example, to indicate the
following cases in which the relation is seen at once and is perfectly clear:—
| K = 39 |
Rb = 85 |
Cs = 133 |
| Ca = 40 |
Sr = 87 |
Ba = 137 |
whereas with the equivalents then in use—
| K = 39 |
Rb = 85 |
Cs = 133 |
| Ca = 20 |
Sr = 43·5 |
Ba = 68·5 |
the consecutiveness of change in atomic weight, which with the true values
is so evident, completely disappears.
Secondly, it had become evident during the period 1860–70, and even
during the preceding decade, that the relations between the atomic weights
of analogous elements were governed by some general and simple laws.
Cooke, Cremers, Gladstone, Gmelin, Lenssen, Pettenkofer, and especially
Dumas, had already established many facts bearing on that view. Thus
Dumas compared the following groups of analogous elements with organic
radicles:—
[474]
| |
|
Diff. |
|
|
Diff. |
|
|
Diff. |
|
|
Diff. |
| |
|
|
Mg = 12 |
|
|
P = 81 |
|
|
O = 8 |
|
|
| |
|
|
|
⎬ |
8 |
|
⎬ |
44 |
|
⎬ |
8 |
| Li = 7 |
|
|
Ca = 20 |
|
|
As= 75 |
|
|
S = 16 |
|
|
| |
⎬ |
16 |
|
⎬ |
3 × 8 |
|
⎬ |
44 |
|
⎬ |
3 × 8 |
| Na = 23 |
|
|
Sr = 44 |
|
|
Sb = 119 |
|
|
Se = 40 |
|
|
| |
⎬ |
16 |
|
⎬ |
3 × 8 |
|
⎬ |
2 × 44 |
|
⎬ |
3 × 8 |
| K = 39 |
|
|
Ba = 68 |
|
|
Bi = 207 |
|
|
Te = 64 |
|
|
and pointed out some really striking relationships, such as the following:—
| F = 19. |
| Cl = 35·5 = 19 + 16·5. |
| Br = 80 = 19 + 2 × 16·5 + 28. |
| I = 127 = 2 x 19 + 2 × 16·5 + 2 × 28. |
A. Strecker, in his work ‘Theorien und Experimente zur Bestimmung
der Atomgewichte der Elemente’ (Braunschweig, 1859), after summarising
the data relating to the subject, and pointing out the remarkable series of
equivalents—
Cr = 26·2 Mn = 27·6 Fe = 28 Ni = 29 Co = 30 Cu = 31·7 Zn = 32·5
remarks that: ‘It is hardly probable that all the above-mentioned relations
between the atomic weights (or equivalents) of chemically analogous elements
are merely accidental. We must, however, leave to the future the discovery
of the law of the relations which appears in these figures.’[1]
In such attempts at arrangement and in such views are to be recognised
the real forerunners of the periodic law; the ground was prepared for it
between 1860 and 1870, and that it was not expressed in a determinate form
before the end of the decade may, I suppose, be ascribed to the fact that only
analogous elements had been compared. The idea of seeking for a relation
between the atomic weights of all the elements was foreign to the ideas then
current, so that neither the vis tellurique of De Chancourtois, nor the law of
octaves of Newlands, could secure anybody's attention. And yet both De
Chancourtois and Newlands like Dumas and Strecker, more than Lenssen
and Pettenkofer, had made an approach to the periodic law and had discovered
its germs. The solution of the problem advanced but slowly, because
the facts, but not the law, stood foremost in all attempts; and the law could
not awaken a general interest so long as elements, having no apparent connection
with each other, were included in the same octave, as for example:—
| 1st octave of Newlands |
H |
F |
Cl |
Co & Ni |
Br |
Pd |
Ir |
Pt & Ir |
| 7th Ditto |
O |
S |
Fe |
Se |
Rh & Ru |
Te |
Au |
Os or Th |
Analogies of the above order seemed quite accidental, and the more so as
the octave contained occasionally ten elements instead of eight, and when two[475]
such elements as Ba and V, Co and Ni, or Rh and Ru, occupied one place in
the octave.[2] Nevertheless, the fruit was ripening, and I now see clearly that
Strecker, De Chancourtois, and Newlands stood foremost in the way towards
the discovery of the periodic law, and that they merely wanted the boldness
necessary to place the whole question at such a height that its reflection on
the facts could be clearly seen.
A third circumstance which revealed the periodicity of chemical elements
was the accumulation, by the end of the sixties, of new information respecting
the rare elements, disclosing their many-sided relations to the other elements
and to each other. The researches of Marignac on niobium, and those of
Roscoe on vanadium, were of special moment. The striking analogies between
vanadium and phosphorus on the one hand, and between vanadium and
chromium on the other, which became so apparent in the investigations connected
with that element, naturally induced the comparison of V = 51 with
Cr = 52, Nb = 94 with Mo = 96, and Ta = 192 with W = 194; while, on the
other hand, P = 31 could be compared with S = 32, As = 75 with Se = 79, and
Sb = 120 with Te = 125. From such approximations there remained but one
step to the discovery of the law of periodicity.
The law of periodicity was thus a direct outcome of the stock of generalisations
and established facts which had accumulated by the end of the decade
1860–1870; it is an embodiment of those data in a more or less systematic
expression. Where, then, lies the secret of the special importance which has
since been attached to the periodic law, and has raised it to the position of a
generalisation which has already given to chemistry unexpected aid, and
which promises to be far more fruitful in the future and to impress upon
several branches of chemical research a peculiar and original stamp? The
remaining part of my communication will be an attempt to answer this
question.
In the first place we have the circumstance that, as soon as the law made
its appearance, it demanded a revision of many facts which were considered
by chemists as fully established by existing experience. I shall return, later
on, briefly to this subject, but I wish now to remind you that the periodic
law, by insisting on the necessity for a revision of supposed facts, exposed
itself at once to destruction in its very origin. Its first requirements, however,
have been almost entirely satisfied during the last 20 years; the supposed
facts have yielded to the law, thus proving that the law itself was a
legitimate induction from the verified facts. But our inductions from data
have often to do with such details of a science so rich in facts, that only
generalisations which cover a wide range of important phenomena can attract
general attention. What were the regions touched on by the periodic law?
This is what we shall now consider.
The most important point to notice is, that periodic functions, used for
the purpose of expressing changes which are dependent on variations of time
and space, have been long known. They are familiar to the mind when we
have to deal with motion in closed cycles, or with any kind of deviation from[476]
a stable position, such as occurs in pendulum-oscillations. A like periodic
function became evident in the case of the elements, depending on the mass
of the atom. The primary conception of the masses of bodies, or of the masses
of atoms, belongs to a category which the present state of science forbids us
to discuss, because as yet we have no means of dissecting or analysing the
conception. All that was known of functions dependent on masses derived
its origin from Galileo and Newton, and indicated that such functions
either decrease or increase with the increase of mass, like the attraction of
celestial bodies. The numerical expression of the phenomena was always
found to be proportional to the mass, and in no case was an increase of mass
followed by a recurrence of properties such as is disclosed by the periodic law
of the elements. This constituted such a novelty in the study of the phenomena
of nature that, although it did not lift the veil which conceals the true conception
of mass, it nevertheless indicated that the explanation of that conception
must be searched for in the masses of the atoms; the more so, as all masses
are nothing but aggregations, or additions, of chemical atoms which would be
best described as chemical individuals. Let me remark, by the way, that
though the Latin word ‘individual’ is merely a translation of the Greek word
‘atom,’ nevertheless history and custom have drawn a sharp distinction
between the two words, and the present chemical conception of atoms is
nearer to that defined by the Latin word than by the Greek, although this
latter also has acquired a special meaning which was unknown to the classics.
The periodic law has shown that our chemical individuals display a harmonic
periodicity of properties dependent on their masses. Now natural science
has long been accustomed to deal with periodicities observed in nature, to
seize them with the vice of mathematical analysis, to submit them to the
rasp of experiment. And these instruments of scientific thought would
surely, long since, have mastered the problem connected with the chemical
elements, were it not for a new feature which was brought to light by the
periodic law, and which gave a peculiar and original character to the periodic
function.
If we mark on an axis of abscissæ a series of lengths proportional to
angles, and trace ordinates which are proportional to sines or other trigonometrical
functions, we get periodic curves of a harmonic character. So it
might seem, at first sight, that with the increase of atomic weights the function
of the properties of the elements should also vary in the same harmonious
way. But in this case there is no such continuous change as in the curves
just referred to, because the periods do not contain the infinite number of
points constituting a curve, but a finite number only of such points. An
example will better illustrate this view. The atomic weights—
Ag = 108 Cd = 112 In = 113 Sn = 118 Sb = 120
Te = 125 I = 127
steadily increase, and their increase is accompanied by a modification of
many properties which constitutes the essence of the periodic law. Thus,
for example, the densities of the above elements decrease steadily, being
respectively—
10·5 8·6 7·4 7·2 6·7 6·4 4·9
[477]
while their oxides contain an increasing quantity of oxygen—
Ag2O Cd2O2 In2O3 Sn2O4 Sb2O5 Te2O6 I2O7
But to connect by a curve the summits of the ordinates expressing any
of these properties would involve the rejection of Dalton's law of multiple
proportions. Not only are there no intermediate elements between silver,
which gives AgCl, and cadmium, which gives CdCl2, but, according to the
very essence of the periodic law, there can be none; in fact a uniform curve
would be inapplicable in such a case, as it would lead us to expect elements
possessed of special properties at any point of the curve. The periods of the
elements have thus a character very different from those which are so simply
represented by geometers. They correspond to points, to numbers, to sudden
changes of the masses, and not to a continuous evolution. In these sudden
changes destitute of intermediate steps or positions, in the absence of
elements intermediate between, say, silver and cadmium, or aluminium
and silicon, we must recognise a problem to which no direct application
of the analysis of the infinitely small can be made. Therefore, neither the
trigonometrical functions proposed by Ridberg and Flavitzky, nor the pendulum-oscillations
suggested by Crookes, nor the cubical curves of the Rev.
Mr. Haughton, which have been proposed for expressing the periodic law,
from the nature of the case, can represent the periods of the chemical
elements. If geometrical analysis is to be applied to this subject, it will require
to be modified in a special manner. It must find the means of representing
in a special way, not only such long periods as that comprising
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br,
but short periods like the following:—
Na Mg Al Si P S Cl.
In the theory of numbers only do we find problems analogous to ours,
and two attempts at expressing the atomic weights of the elements by algebraic
formulæ seem to be deserving of attention, although neither of them
can be considered as a complete theory, nor as promising finally to solve the
problem of the periodic law. The attempt of E. J. Mills (1886) does not
even aspire to attain this end. He considers that all atomic weights can be
expressed by a logarithmic function,
15(n - 0·9375t),
in which the variables n and t are whole numbers. Thus, for oxygen, n = 2,
and t = 1, whence its atomic weight is = 15·94; in the case of chlorine,
bromine, and iodine, n has respective values of 3, 6, and 9, whilst t = 7, 6,
and 9; in the case of potassium, rubidium, and cæsium, n = 4, 6, and 9, and
t = 14, 18, and 20.
Another attempt was made in 1888 by B. N. Tchitchérin. Its author
places the problem of the periodic law in the first rank, but as yet he has
investigated the alkali metals only. Tchitchérin first noticed the simple[478]
relations existing between the atomic volumes of all alkali metals; they
can be expressed, according to his views, by the formula
A(2 - 0·00535An),
where A is the atomic weight, and n is equal to 8 for lithium and sodium, to
4 for potassium, to 3 for rubidium, and to 2 for cæsium. If n remained equal
to 8 during the increase of A, the volume would become zero at A = 46⅔,
and it would reach its maximum at A = 23⅓. The close approximation of
the number 46⅔ to the differences between the atomic weights of analogous
elements (such as Cs - Rb, I - Br, and so on); the close correspondence of
the number 23⅓ to the atomic weight of sodium; the fact of n being necessarily
a whole number, and several other aspects of the question, induce
Tchitchérin to believe that they afford a clue to the understanding of the
nature of the elements; we must, however, await the full development of
his theory before pronouncing judgment on it. What we can at present only
be certain of is this: that attempts like the two above named must be repeated
and multiplied, because the periodic law has clearly shown that the
masses of the atoms increase abruptly, by steps, which are clearly connected
in some way with Dalton's law of multiple proportions; and because the
periodicity of the elements finds expression in the transition from RX to
RX2, RX3, RX4, and so on till RX8, at which point, the energy of the combining
forces being exhausted, the series begins anew from RX to RX2, and
so on.
While connecting by new bonds the theory of the chemical elements with
Dalton's theory of multiple proportions, or atomic structure of bodies, the
periodic law opened for natural philosophy a new and wide field for speculation.
Kant said that there are in the world ‘two things which never cease
to call for the admiration and reverence of man: the moral law within
ourselves, and the stellar sky above us.’ But when we turn our thoughts
towards the nature of the elements and the periodic law, we must add a third
subject, namely, ‘the nature of the elementary individuals which we discover
everywhere around us.’ Without them the stellar sky itself is inconceivable;
and in the atoms we see at once their peculiar individualities, the infinite
multiplicity of the individuals, and the submission of their seeming
freedom to the general harmony of Nature.
Having thus indicated a new mystery of Nature, which does not yet yield
to rational conception, the periodic law, together with the revelations of
spectrum analysis, have contributed to again revive an old but remarkably
long-lived hope—that of discovering, if not by experiment, at least by a
mental effort, the primary matter—which had its genesis in the minds of
the Grecian philosophers, and has been transmitted, together with many
other ideas of the classic period, to the heirs of their civilisation. Having
grown, during the times of the alchemists up to the period when experimental
proof was required, the idea has rendered good service; it induced those
careful observations and experiments which later on called into being the
works of Scheele, Lavoisier, Priestley, and Cavendish. It then slumbered
awhile, but was soon awakened by the attempts either to confirm or to refute
the ideas of Prout as to the multiple proportion relationship of the atomic[479]
weights of all the elements. And once again the inductive or experimental
method of studying Nature gained a direct advantage from the old Pythagorean
idea: because atomic weights were determined with an accuracy
formerly unknown. But again the idea could not stand the ordeal of experimental
test, yet the prejudice remains and has not been uprooted, even by
Stas; nay, it has gained a new vigour, for we see that all which is imperfectly
worked out, new and unexplained, from the still scarcely studied rare metals
to the hardly perceptible nebulæ, have been used to justify it. As soon as
spectrum analysis appears as a new and powerful weapon of chemistry, the
idea of a primary matter is immediately attached to it. From all sides we
see attempts to constitute the imaginary substance helium[3] the so much
longed for primary matter. No attention is paid to the circumstance that
the helium line is only seen in the spectrum of the solar protuberances, so
that its universality in Nature remains as problematic as the primary matter
itself; nor to the fact that the helium line is wanting amongst the Fraunhofer
lines of the solar spectrum, and thus does not answer to the brilliant
fundamental conception which gives its real force to spectrum analysis.
And finally, no notice is even taken of the indubitable fact that the brilliancies
of the spectral lines of the simple substances vary under different temperatures
and pressures; so that all probabilities are in favour of the helium
line simply belonging to some long since known element placed under such
conditions of temperature, pressure, and gravity as have not yet been realised
in our experiments. Again, the idea that the excellent investigations of
Lockyer of the spectrum of iron can be interpreted in favour of the compound
nature of that element, evidently must have arisen from some misunderstanding.
The spectrum of a compound certainly does not appear as a
sum of the spectra of its components; and therefore the observations of
Lockyer can be considered precisely as a proof that iron undergoes no other
changes at the temperature of the sun than those which it experiences in the
voltaic arc—provided the spectrum of iron is preserved. As to the shifting
of some of the lines of the spectrum of iron while the other lines maintain
their positions, it can be explained, as shown by M. Kleiber (‘Journal of the
Russian Chemical and Physical Society,’ 1885, 147), by the relative motion
of the various strata of the sun's atmosphere, and by Zöllner's laws of the
relative brilliancies of different lines of the spectrum. Moreover, it ought
not to be forgotten that if iron were really proved to consist of two or more
unknown elements, we should simply have an increase in the number of our
elements—not a reduction, and still less a reduction of all of them to one
single primary matter.
Feeling that spectrum analysis will not yield a support to the Pythagorean
conception, its modern promoters are so bent upon its being confirmed by
the periodic law, that the illustrious Berthelot, in his work ‘Les origines de
l'Alchimie,’ 1885, 313, has simply mixed up the fundamental idea of the law
of periodicity with the ideas of Prout, the alchemists, and Democritus about
primary matter.[4] But the periodic law, based as it is on the solid and wholesome[480]
ground of experimental research, has been evolved independently of
any conception as to the nature of the elements; it does not in the least
originate in the idea of a unique matter; and it has no historical connection
with that relic of the torments of classical thought, and therefore it
affords no more indication of the unity of matter or of the compound character
of our elements, than the law of Avogadro, or the law of specific heats, or
even the conclusions of spectrum analysis. None of the advocates of a
unique matter have ever tried to explain the law from the standpoint of ideas
taken from a remote antiquity when it was found convenient to admit the
existence of many gods—and of a unique matter.
When we try to explain the origin of the idea of a unique primary
matter, we easily trace that in the absence of inductions from experiment it
derives its origin from the scientifically philosophical attempt at discovering
some kind of unity in the immense diversity of individualities which we see
around. In classical times such a tendency could only be satisfied by conceptions
about the immaterial world. As to the material world, our ancestors
were compelled to resort to some hypothesis, and they adopted the idea of
unity in the formative material, because they were not able to evolve the
conception of any other possible unity in order to connect the multifarious
relations of matter. Responding to the same legitimate scientific tendency,
natural science has discovered throughout the universe a unity of plan, a
unity of forces, and a unity of matter, and the convincing conclusions of
modern science compel every one to admit these kinds of unity. But while
we admit unity in many things, we none the less must also explain the
individuality and the apparent diversity which we cannot fail to trace everywhere.
It has been said of old, ‘Give us a fulcrum, and it will become easy to
displace the earth.’ So also we must say, ‘Give us something that is individualised,
and the apparent diversity will be easily understood.’ Otherwise, how
could unity result in a multitude?
After a long and painstaking research, natural science has discovered the
individualities of the chemical elements, and therefore it is now capable not
only of analysing, but also of synthesising; it can understand and grasp
generality and unity, as well as the individualised and the multifarious.
The general and universal, like time and space, like force and motion, vary uniformly;
the uniform admit of interpolations, revealing every intermediate
phase. But the multitudinous, the individualised—such as ourselves, or the
chemical elements, or the members of a peculiar periodic function of the
elements, or Dalton's multiple proportions—is characterised in another
way: we see in it, side by side with a connecting general principle, leaps,
breaks of continuity, points which escape from the analysis of the infinitely
small—an absence of complete intermediate links. Chemistry has found an
answer to the question as to the causes of multitudes; and while retaining
the conception of many elements, all submitted to the discipline of a general
law, it offers an escape from the Indian Nirvana—the absorption in the
universal, replacing it by the individualised. However, the place for individuality[481]
is so limited by the all-grasping, all-powerful universal, that it is
merely a point of support for the understanding of multitude in unity.
Having touched upon the metaphysical bases of the conception of a
unique matter which is supposed to enter into the composition of all bodies.
I think it necessary to dwell upon another theory, akin to the above conception—the
theory of the compound character of the elements now admitted by
some—and especially upon one particular circumstance which, being related
to the periodic law, is considered to be an argument in favour of that hypothesis.
Dr. Pelopidas, in 1883, made a communication to the Russian Chemical
and Physical Society on the periodicity of the hydrocarbon radicles, pointing
out the remarkable parallelism which was to be noticed in the change of
properties of hydrocarbon radicles and elements when classed in groups.
Professor Carnelley, in 1886, developed a similar parallelism. The idea of
M. Pelopidas will be easily understood if we consider the series of hydrocarbon
radicles which contain, say, 6 atoms of carbon:—
| I. |
II. |
III. |
IV. |
V. |
VI. |
VII. |
VIII. |
| C6H13 |
C6H12 |
C6H11 |
C6H10 |
C6H9 |
C6H8 |
C6H7 |
C6H6 |
The first of these radicles, like the elements of the 1st group, combines with
Cl, OH, and so on, and gives the derivatives of hexyl alcohol, C6H13(OH);
but, in proportion as the number of hydrogen atoms decreases, the capacity
of the radicles of combining with, say, the halogens increases. C6H12 already
combines with 2 atoms of chlorine; C6H11, with 3 atoms, and so on. The
last members of the series comprise the radicles of acids: thus C6H8, which
belongs to the 6th group, gives, like sulphur, a bibasic acid, C6H8O2(OH)2,
which is homologous with oxalic acid. The parallelism can be traced still
further, because C6H5 appears as a monovalent radicle of benzene, and with
it begins a new series of aromatic derivatives, so analogous to the derivatives of
the aliphatic series. Let me also mention another example from among those
which have been given by M. Pelopidas. Starting from the alkaline radicle
of monomethylammonium, N(CH3)H3, or NCH6, which presents many
analogies with the alkaline metals of the 1st group, he arrives, by successively
diminishing the number of the atoms of hydrogen, at a 7th group which
contains cyanogen, CN, which has long since been compared to the halogens
of the 7th group.
The most important consequence which, in my opinion, can be drawn
from the above comparison is that the periodic law, so apparent in the
elements, has a wider application than might appear at first sight; it opens
up a new vista of chemical evolutions. But, while admitting the fullest
parallelism between the periodicity of the elements and that of the compound
radicles, we must not forget that in the periods of the hydrocarbon radicles
we have a decrease of mass as we pass from the representatives of the first
group to the next, while in the periods of the elements the mass increases
during the progression. It thus becomes evident that we cannot speak of an
identity of periodicity in both cases, unless we put aside the ideas of mass
and attraction, which are the real corner-stones of the whole of natural
science, and even enter into those very conceptions of simple substances which[482]
came to light a full hundred years later than the immortal principles of
Newton.[5]
From the foregoing, as well as from the failures of so many attempts at
finding in experiment and speculation a proof of the compound character of
the elements and of the existence of primordial matter, it is evident, in my
opinion, that this theory must be classed among mere utopias. But utopias
can only be combated by freedom of opinion, by experiment, and by new
utopias. In the republic of scientific theories freedom of opinions is guaranteed.
It is precisely that freedom which permits me to criticise openly the
widely-diffused idea as to the unity of matter in the elements. Experiments
and attempts at confirming that idea have been so numerous that it really
would be instructive to have them all collected together, if only to serve as a
warning against the repetition of old failures. And now as to new utopias
which may be helpful in the struggle against the old ones, I do not think it
quite useless to mention a phantasy of one of my students who imagined that
the weight of bodies does not depend upon their mass, but upon the character
of the motion of their atoms. The atoms, according to this new utopian, may
all be homogeneous or heterogeneous, we know not which; we know them
in motion only, and that motion they maintain with the same persistence as
the stellar bodies maintain theirs. The weights of atoms differ only in consequence
of their various modes and quantity of motion; the heaviest atoms
may be much simpler than the lighter ones: thus an atom of mercury may
be simpler than an atom of hydrogen—the manner in which it moves causes
it to be heavier. My interlocutor even suggested that the view which
attributes the greater complexity to the lighter elements finds confirmation
in the fact that the hydrocarbon radicles mentioned by Pelopidas, while
becoming lighter as they lose hydrogen, change their properties periodically
in the same manner as the elements change theirs, according as the atoms
grow heavier.
The French proverb, La critique est facile, mais l'art est difficile, however,
may well be reversed in the case of all such ideal views, as it is much
easier to formulate than to criticise them. Arising from the virgin soil of
newly-established facts, the knowledge relating to the elements, to their
masses, and to the periodic changes of their properties has given a motive
for the formation of utopian hypotheses, probably because they could not be
foreseen by the aid of any of the various metaphysical systems, and exist,
like the idea of gravitation, as an independent outcome of natural science,
requiring the acknowledgment of general laws, when these have been established
with the same degree of persistency as is indispensable for the acceptance
of a thoroughly established fact. Two centuries have elapsed since the
theory of gravitation was enunciated, and although we do not understand its
cause, we still must regard gravitation as a fundamental conception of natural
philosophy, a conception which has enabled us to perceive much more than
the metaphysicians did or could with their seeming omniscience. A hundred[483]
years later the conception of the elements arose; it made chemistry what it
now is; and yet we have advanced as little in our comprehension of simple
substances since the times of Lavoisier and Dalton as we have in our understanding
of gravitation. The periodic law of the elements is only twenty
years old; it is not surprising, therefore, that, knowing nothing about the
causes of gravitation and mass, or about the nature of the elements, we do
not comprehend the rationale of the periodic law. It is only by collecting
established laws—that is, by working at the acquirement of truth—that we
can hope gradually to lift the veil which conceals from us the causes of the
mysteries of Nature and to discover their mutual dependency. Like the
telescope and the microscope, laws founded on the basis of experiment are
the instruments and means of enlarging our mental horizon.
In the remaining part of my communication I shall endeavour to show,
and as briefly as possible, in how far the periodic law contributes to enlarge
our range of vision. Before the promulgation of this law the chemical
elements were mere fragmentary, incidental facts in Nature; there was no
special reason to expect the discovery of new elements, and the new ones
which were discovered from time to time appeared to be possessed of quite
novel properties. The law of periodicity first enabled us to perceive undiscovered
elements at a distance which formerly was inaccessible to chemical
vision; and long ere they were discovered new elements appeared before our
eyes possessed of a number of well-defined properties. We now know three
cases of elements whose existence and properties were foreseen by the instrumentality
of the periodic law. I need but mention the brilliant discovery of
gallium, which proved to correspond to eka-aluminium of the periodic law, by
Lecoq de Boisbaudran; of scandium, corresponding to ekaboron, by Nilson;
and of germanium, which proved to correspond in all respects to ekasilicon,
by Winkler. When, in 1871, I described to the Russian Chemical Society
the properties, clearly defined by the periodic law, which such elements
ought to possess, I never hoped that I should live to mention their discovery
to the Chemical Society of Great Britain as a confirmation of the exactitude
and the generality of the periodic law. Now that I have had the happiness
of doing so, I unhesitatingly say that, although greatly enlarging our vision,
even now the periodic law needs further improvements in order that it may
become a trustworthy instrument in further discoveries.[6]
I will venture to allude to some other matters which chemistry has discerned
by means of its new instrument, and which it could not have made[484]
out without a knowledge of the law of periodicity, and I will confine myself
to simple substances and to oxides.
Before the periodic law was formulated the atomic weights of the elements
were purely empirical numbers, so that the magnitude of the equivalent, and
the atomicity, or the value in substitution possessed by an atom, could only
be tested by critically examining the methods of determination, but never
directly by considering the numerical values themselves; in short, we were
compelled to move in the dark, to submit to the facts, instead of being masters
of them. I need not recount the methods which permitted the periodic law
at last to master the facts relating to atomic weights, and I would merely
call to mind that it compelled us to modify the valencies of indium and
cerium, and to assign to their compounds a different molecular composition.
Determinations of the specific heats of these two metals fully confirmed the
change. The trivalency of yttrium, which makes us now represent its oxide
as Y2O3 instead of as YO, was also foreseen (in 1870) by the periodic law, and
it has now become so probable that Clève, and all other subsequent investigators
of the rare metals, have not only adopted it, but have also applied it
without any new demonstration to substances so imperfectly known as those
of the cerite and gadolinite group, especially since Hillebrand determined the
specific heats of lanthanum and didymium and confirmed the expectations
suggested by the periodic law. But here, especially in the case of didymium,
we meet with a series of difficulties long since foreseen through the periodic
law, but only now becoming evident, and chiefly arising from the relative
rarity and insufficient knowledge of the elements which usually accompany
didymium.
Passing to the results obtained in the case of the rare elements beryllium,
scandium, and thorium, it is found that these have many points of contact
with the periodic law. Although Avdéeff long since proposed the magnesia
formula to represent beryllium oxide, yet there was so much to be said in
favour of the alumina formula, on account of the specific heat of the metals
and the isomorphism of the two oxides, that it became generally adopted
and seemed to be well established. The periodic law, however, as Brauner
repeatedly insisted (‘Berichte,’ 1878, 872; 1881, 53), was against the formula
Be2O3; it required the magnesia formula BeO—that is, an atomic weight
of 9—because there was no place in the system for an element like beryllium
having an atomic weight of 13·5. This divergence of opinion lasted for
years, and I often heard that the question as to the atomic weight of beryllium
threatened to disturb the generality of the periodic law, or, at any rate, to
require some important modifications of it. Many forces were operating in
the controversy regarding beryllium, evidently because a much more important
question was at issue than merely that involved in the discussion of
the atomic weight of a relatively rare element: and during the controversy
the periodic law became better understood, and the mutual relations of the
elements became more apparent than ever before. It is most remarkable that
the victory of the periodic law was won by the researches of the very observers
who previously had discovered a number of facts in support of the trivalency
of beryllium. Applying the higher law of Avogadro, Nilson and
Petterson have finally shown that the density of the vapour of the beryllium[485]
chloride, BeCl2, obliges us to regard beryllium as bivalent in
conformity with the periodic law.[7] I consider the confirmation of Avdéeff's
and Brauner's view as important in the history of the periodic law as the
discovery of scandium, which, in Nilson's hands, confirmed the existence of
ekaboron.
The circumstance that thorium proved to be quadrivalent, and Th = 232,
in accordance with the views of Chydenius and the requirements of the
periodic law, passed almost unnoticed, and was accepted without opposition,
and yet both thorium and uranium are of great importance in the periodic
system, as they are its last members, and have the highest atomic weights of
all the elements.
The alteration of the atomic weight of uranium from U = 120 into U = 240
attracted more attention, the change having been made on account of the
periodic law, and for no other reason. Now that Roscoe, Rammelsberg,
Zimmermann, and several others have admitted the various claims of the
periodic law in the case of uranium, its high atomic weight is received without
objection, and it endows that element with a special interest.
While thus demonstrating the necessity for modifying the atomic weights
of several insufficiently known elements, the periodic law enabled us also to
detect errors in the determination of the atomic weights of several elements
whose valencies and true position among other elements were already well
known. Three such cases are especially noteworthy: those of tellurium,
titanium and platinum. Berzelius had determined the atomic weight of
tellurium to be 128, while the periodic law claimed for it an atomic weight
below that of iodine, which had been fixed by Stas at 126·5, and which was
certainly not higher than 127. Brauner then undertook the investigation,
and he has shown that the true atomic weight of tellurium is lower than that
of iodine, being near to 125. For titanium the extensive researches of
Thorpe have confirmed the atomic weight of Ti = 48, indicated by the law,
and already foreseen by Rose, but contradicted by the analyses of Pierre and
several other chemists. An equally brilliant confirmation of the expectations
based on the periodic law has been given in the case of the series osmium,
iridium, platinum, and gold. At the time of the promulgation of the periodic
law, the determinations of Berzelius, Rose, and many others gave the following
figures:—
Os = 200 Ir = 197; Pt = 198; Au = 196.
[486]
The expectations of the periodic law[8] have been confirmed, first, by new
determinations of the atomic weight of platinum (by Seubert, Dittmar, and
M'Arthur, which proved to be near to 196 (taking O = 16, as proposed by
Marignac, Brauner, and others); secondly, by Seubert having proved that
the atomic weight of osmium is really lower than that of platinum, being
near to 191; and thirdly, by the investigations of Krüss, Thorpe and
Laurie, proving that the atomic weight of gold exceeds that of platinum,
and approximates to 197. The atomic weights which were thus found to
require correction were precisely those which the periodic law had indicated
as affected with errors; and it has been proved, therefore, that the periodic
law affords a means of testing experimental results. If we succeed in discovering
the exact character of the periodic relationships between the
increments in atomic weights of allied elements discussed by Ridberg in
1885, and again by Bazaroff in 1887, we may expect that our instrument
will give us the means of still more closely controlling the experimental data
relating to atomic weights.
Let me next call to mind that, while disclosing the variation of chemical
properties,[9] the periodic law, has also enabled us to systematically discuss
many of the physical properties of elementary bodies, and to show that these
properties are also subject to the law of periodicity. At the Moscow Congress
of Russian Naturalists in August, 1869, I dwelt upon the relations which
existed between density and the atomic weight of the elements. The following
year Professor Lothar Meyer, in his well-known paper,[10] studied the
same subject in more detail, and thus contributed to spread information
about the periodic law. Later on, Carnelley, Laurie, L. Meyer, Roberts-Austen,
and several others applied the periodic system to represent the order
in the changes of the magnetic properties of the elements, their melting
points, the heats of formation of their haloid compounds, and even of such
mechanical properties as the coefficient of elasticity, the breaking stress, &c.,
&c. These deductions, which have received further support in the discovery
of new elements endowed not only with chemical but even with physical
properties, which were foreseen by the law of periodicity, are well known;
so I need not dwell upon the subject, and may pass to the consideration of
oxides.[11]
[487]
In indicating that the gradual increase of the power of elements of combining
with oxygen is accompanied by a corresponding decrease in their
power of combining with hydrogen, the periodic law has shown that there is
a limit of oxidation, just as there is a well-known limit to the capacity of
elements for combining with hydrogen. A single atom of an element combines
with at most four atoms of either hydrogen or oxygen; and while CH4
and SiH4 represent the highest hydrides, so RuO4 and OsO4 are the highest
oxides. We are thus led to recognise types of oxides, just as we have had to
recognise types of hydrides.[12]
The periodic law has demonstrated that the maximum extent to which
different non-metals enter into combination with oxygen is determined by the
extent to which they combine with hydrogen, and that the sum of the number
of equivalents of both must be equal to 8. Thus chlorine, which combines
with 1 atom or 1 equivalent of hydrogen, cannot fix more than 7 equivalents
of oxygen, giving Cl2O7; while sulphur, which fixes 2 equivalents of hydrogen,
cannot combine with more than 6 equivalents or 3 atoms of oxygen. It thus
becomes evident that we cannot recognise as a fundamental property of the
elements the atomic valencies deduced from their hydrides; and that we
must modify, to a certain extent, the theory of atomicity if we desire to raise
it to the dignity of a general principle capable of affording an insight into the
constitution of all compound molecules. In other words, it is only to carbon,
which is quadrivalent with regard both to oxygen and hydrogen, that we can
apply the theory of constant valency and of bond, by means of which so many
still endeavour to explain the structure of compound molecules. But I should
go too far if I ventured to explain in detail the conclusions which can be
drawn from the above considerations. Still, I think it necessary to dwell
upon one particular fact which must be explained from the point of view of
the periodic law in order to clear the way to its extension in that particular
direction.
The higher oxides yielding salts the formation of which was foreseen by
the periodic system—for instance, in the short series beginning with sodium—
Na2O, MgO, Al2O3, SiO2, P2O5, SO3, Cl2O7,
must be clearly distinguished from the higher degrees of oxidation which correspond
to hydrogen peroxide and bear the true character of peroxides. Peroxides
such as Na2O2, BaO2, and the like have long been known. Similar[488]
peroxides have also recently become known in the case of chromium, sulphur,
titanium, and many other elements, and I have sometimes heard it said that
discoveries of this kind weaken the conclusions of the periodic law in so far
as it concerns the oxides. I do not think so in the least, and I may remark,
in the first place, that all these peroxides are endowed with certain properties
obviously common to all of them, which distinguish them from the actual,
higher, salt-forming oxides, especially their easy decomposition by means of
simple contact agencies; their incapability of forming salts of the common
type; and their capability of combining with other peroxides (like the faculty
which hydrogen peroxide possesses of combining with barium peroxide, discovered
by Schoene). Again, we remark that some groups are especially
characterised by their capacity of generating peroxides. Such is, for instance,
the case in the sixth group, where we find the well-known peroxides of
sulphur, chromium, and uranium; so that further investigation of peroxides
will probably establish a new periodic function, foreshadowing that molybdenum
and tungsten will assume peroxide forms with comparative readiness.
To appreciate the constitution of such peroxides, it is enough to notice that
the peroxide form of sulphur (so-called persulphuric acid) stands in the same
relation to sulphuric acid as hydrogen peroxide stands to water:—
H(OH), or H2O, responds to (OH)(OH), or H2O2,
and so also—
H(HSO4), or H2SO4, responds to (HSO4)(HSO4), or H2S2O8.
Similar relations are seen everywhere, and they correspond to the principle
of substitutions which I long since endeavoured to represent as one of the
chemical generalisations called into life by the periodic law. So also
sulphuric acid, if considered with reference to hydroxyl, and represented as
follows:—
HO(SO2OH),
has its corresponding compound in dithionic acid—
(SO2OH)(SO2OH), or H2S2O6.
Therefore, also, phosphoric acid, HO(POH2O2), has, in the same sense, its
corresponding compound in the subphosphoric acid of Saltzer:—
(POH2O2)(POH2O2), or H4P2O6;
and we must suppose that the peroxide compound corresponding to phosphoric
acid, if it be discovered, will have the following structure:—
(H2PO4)2 or H4P2O8 = 2H2O + 2PO3.[13]
So far as is known at present, the highest form of peroxides is met with in[489]
the peroxide of uranium, UO4, prepared by Fairley;[14] while OsO4 is the
highest oxide giving salts. The line of argument which is inspired by the
periodic law, so far from being weakened by the discovery of peroxides, is
thus actually strengthened, and we must hope that a further exploration of
the region under consideration will confirm the applicability to chemistry
generally of the principles deduced from the periodic law.
Permit me now to conclude my rapid sketch of the oxygen compounds by
the observation that the periodic law is especially brought into evidence in
the case of the oxides which constitute the immense majority of bodies at our
disposal on the surface of the earth.
The oxides are evidently subject to the law, both as regards their chemical
and their physical properties, especially if we take into account the cases of
polymerism which are so obvious when comparing CO2, with SinO2n. In order
to prove this I give the densities s and the specific volumes v of the higher
oxides of two short periods. To render comparison easier, the oxides are all
represented as of the form R2On. In the column headed Δ the differences
are given between the volume of the oxygen compound and that of the parent
element, divided by n—that is, by the number of atoms of oxygen in the
compound:—[15]
| |
s. |
v. |
Δ |
|
s. |
v. |
Δ |
| Na2O |
2·6 |
24 |
-22 |
K2O |
2·7 |
35 |
-55 |
| Mg2O2 |
3·6 |
22 |
-3 |
Ca2O |
3·15 |
36 |
-7 |
| Al2O3 |
4·0 |
26 |
+1·3 |
Sc2O3 |
3·86 |
35 |
0 |
| Si2O4 |
2·65 |
45 |
5·2 |
Li2O4 |
4·2 |
38 |
+5 |
| P2O5 |
2·39 |
59 |
6·2 |
V2O5 |
3·49 |
52 |
6·7 |
| S2O6 |
1·96 |
82 |
8·7 |
Cr2O6 |
2·74 |
73 |
9·5 |
I have nothing to add to these figures, except that like relations appear in
other periods as well. The above relations were precisely those which made
it possible for me to be certain that the relative density of ekasilicon oxide
would be about 4·7; germanium oxide, actually obtained by Winkler, proved,
in fact, to have the relative density 4·703.
The foregoing account is far from being an exhaustive one of all that has
already been discovered by means of the periodic law telescope in the boundless
realms of chemical evolution. Still less is it an exhaustive account of all
that may yet be seen, but I trust that the little which I have said will account[490]
for the philosophical interest attached in chemistry to this law. Although
but a recent scientific generalisation, it has already stood the test of laboratory
verification, and appears as an instrument of thought which has not yet been
compelled to undergo modification; but it needs not only new applications,
but also improvements, further development, and plenty of fresh energy. All
this will surely come, seeing that such an assembly of men of science as the
Chemical Society of Great Britain has expressed the desire to have the history
of the periodic law described in a lecture dedicated to the glorious name
of Faraday.
[491]
APPENDIX III
ARGON, A NEW CONSTITUENT OF THE ATMOSPHERE
Written by PROFESSOR MENDELÉEFF IN FEBRUARY 1895
The remarks made in Chapter V., Note 16 bis respecting the newly discovered
constituent of the atmosphere are here supplemented by data (taken from
the publications of the Royal Society of London) given by the discoverers
Lord Rayleigh and Professor Ramsay in January 1895, together with observations
made by Crookes and Olszewsky upon the same subject.
This gas, which was discovered by Rayleigh and Ramsay in atmospheric
nitrogen, was named argon[1] by them, and upon the supposition of
its being an element, they gave it the symbol A. But its true chemical
nature is not yet fully known, for not only has no compound of it been yet
obtained, but it has not even been brought into any reaction. From all that
is known about it at the present time, we may conclude with the discoverers
that argon belongs to those gases which are permanent constituents of the
atmosphere, and that it is a new element. The latter statement, however,
requires confirmation. We shall presently see, however, that the negative
chemical character of argon (its incapacity to react with any substance), and
the small amount of it present in the atmosphere (about 1¼ per cent. by
volume in the nitrogen of air, and consequently about 1 per cent. by volume
in air), as well as the recent date of its discovery (1894) and the difficulty
of its preparation, are quite sufficient reasons for the incompleteness of the
existing knowledge respecting this element. But since, so far as is yet known,
we are dealing with a normal constituent of the atmosphere[1 bis], the[492]
existing data, notwithstanding their insufficiently definite nature, should
find a place even in such an elementary work as the present, all the more as
the names of Rayleigh, Ramsay, Crookes and Olszewsky, who have worked
upon argon, are among the highest in our science, and their researches among
the most difficult.[2] These researches, moreover, were directed straight to
the goal, which was only partly reached owing to the unusual properties of
argon itself.
When it became known (Chapter V., Note 4 bis) that the nitrogen obtained
from air (by removing the oxygen, moisture and CO2, by various reagents)
has a greater density than that obtained from the various (oxygen, hydrogen
and metallic) compounds of nitrogen, it was a plausible explanation that the
latter contained an admixture of hydrogen, or of some other light gas lowering
the density of the mixture. But such an assumption is refuted not only
by the fact that the nitrogen obtained from its various compounds (after
purification) has always the same density (although the supposed impurities
mixed with it should vary), but also by Rayleigh and Ramsay's experiment
of artificially adding hydrogen to nitrogen, and then passing the mixture over
red-hot oxide of copper, when it was found that the nitrogen regained its
original density, i.e. that the whole of the hydrogen was removed by this
treatment. Therefore the difference in the density of the two varieties of
nitrogen had to be explained by the presence of a heavier gas in admixture
with the nitrogen obtained from the atmosphere. This hypothesis was confirmed
by the fact that Rayleigh and Ramsay having obtained purified nitrogen
(by removing the O2, CO2, and H2O), both from ordinary air and from air
which had been previously subjected to atmolysis, that is which had been
passed through porous tubes (of burnt clay, e.g. pipe-stem), surrounded by a
rarefied space, and so deprived of its lighter constituents (chiefly nitrogen),
found that the nitrogen from the air which had been subjected to atmolysis
was heavier than that obtained from air which had not been so treated. This
experiment showed that the nitrogen of air contains an admixture of a gas
which, being heavier than nitrogen itself,[3] diffuses more slowly than nitrogen[493]
through the porous material. It remained, therefore, to separate this impurity
from the nitrogen. To do this Rayleigh and Ramsay adopted two
methods, converting the nitrogen into solid and liquid substances, either
by absorbing the nitrogen by heated magnesium (Chapter V., Note 6, and
Chapter XIV., Note 14), with the formation of nitride of magnesium, or else
by converting it into nitric acid by the action of electric sparks or the presence
of an excess of air and alkali, as in Cavendish's method.[3 bis] In both cases
the nitrogen entered into reaction, while the heavier gas mixed with it
remained inert, and was thus able to be isolated. That is, the argon could be
separated by these means from the excess of atmospheric nitrogen accompanying
it.[4] As an illustration we will describe how argon was obtained
from the atmospheric nitrogen by means of magnesium.[5] To begin with,
it was discovered that when atmospheric nitrogen was passed through a tube
containing metallic magnesium heated to redness, its specific gravity rose to
14·88. As this showed that part of the gas was absorbed by the magnesium,
a mercury gasometer filled with atmospheric nitrogen was taken, and the
gas drawn over soda-lime, P2O5, heated magnesium[6] and then through
tubes containing red-hot copper oxide, soda-lime and phosphoric anhydride
to a second mercury gasometer. Every time the gas was repassed through
the tubes, it decreased in volume and increased in density. After repeating[494]
this for ten days 1,500 c.c. of gas were reduced to 200 cc., and the density
increased to 16·1 (if that of H2 = 1 and N2 = 14). Further treatment of the
remainder brought the density up to 19·09. After adding a small quantity
of oxygen and repassing the gas through the apparatus, the density rose to
20·0. To obtain argon by this process Ramsay and Rayleigh (employing a
mercury air pump and mercury gasometers) once treated about 150 litres of
atmospheric nitrogen. On another occasion they treated 7,925 c.c. of air by
the oxidation method and obtained 65 c.c. of argon, which corresponds to
0·82 per cent. The density of the argon obtained by this means was nearly
19·7, while that obtained by the magnesium method varied between 19·09
and 20·38.
Thus the first positive and very important fact respecting argon is that
its specific gravity is nearly 20—that is, that it is 20 times heavier than
hydrogen, while nitrogen is only 14 times and oxygen 16 times heavier than
hydrogen. This explains the difference observed by Rayleigh between the
densities of nitrogen obtained from its compounds and from the atmosphere
(Chapter V., Note 4 bis). At 0° and 760 mm. a litre of the former gas weighs
1·2505 grm., while a litre of the latter weighs 1·2572, or taking H = 1, the
density of the first = 13·916, and of the latter = 13·991. If the density of
argon be taken as 20, it is contained in atmospheric nitrogen to the extent of
about 1·23 per cent. by volume, whilst air contains about 0·97 per cent. by
volume.
When argon had been isolated the question naturally arose, was it a new
homogeneous substance having definite properties or was it a mixture of
gases? The former may now be positively asserted, namely, that argon is a
peculiar gas previously unknown to chemistry. Such a conviction is in the
first place established by the fact that argon has a greater number of negative
properties, a smaller capacity for reaction, than any other simple or
compound body known. The most inert gas known is nitrogen, but argon
far exceeds it in this respect. Thus nitrogen is absorbed at a red heat by many
metals, with the formation of nitrides, while argon, as is seen in the mode
of its preparation and by direct experiment, does not possess this property.
Nitrogen, under the action of electric sparks, combines with hydrogen in the
presence of acids and with oxygen in the presence of alkalis, while argon is
unable to do so, as is seen from the method of separation from nitrogen.
Rayleigh and Ramsay also proved that argon is unable to react with chlorine
(dry or moist) either directly or under the action of an electric discharge, or
with phosphorus or sulphur, at a red heat. Sodium, potassium, and tellurium
may be distilled in an atmosphere of argon without change. Fused caustic
soda, incandescent soda-lime, molten nitre, red-hot peroxide of sodium,
and the polysulphides of calcium and sodium also do not react with argon.
Platinum black does not absorb it, and spongy platinum is unable to excite its
reaction with oxygen or chlorine. Aqua regia, bromine water, and a mixture
of hydrochloric acid and KMnO4 were also without action upon argon. Besides
which it is evident from the method of its preparation that it is not acted upon
by red-hot oxide of copper. All these facts exclude any possibility of argon containing
any already known body, and prove it to be the most inert of all the
gases known. But besides these negative points, the independency of argon is[495]
confirmed by four observed positive properties possessed by it, which
are:—
1. The spectrum of argon observed by Crookes under a low pressure (in
Geissler-Plücker tubes) distinguishes it from other gases.[7] It was proved
by this means that the argon obtained by means of magnesium is identical
with that which remains after the conversion of the atmospheric nitrogen
into nitric acid. Like nitrogen, argon presents two spectra produced at
different potentials of the induced current, one being orange-red, the other
steel-blue; the latter is obtained under a higher degree of rarefaction and
with a battery of Leyden jars. Both the spectra of argon (in contradistinction
to those of nitrogen) are distinguished by clearly defined lines.[8] The red
(ordinary) spectrum of argon has two particularly brilliant and characteristic
red lines (not far from the bright red line of lithium, on the opposite side to
the orange band) having wave-lengths 705·64 and 696·56 (see Vol. I.,
p. 565). Between these bright lines there are in addition lines with wave
lengths 603·8, 565·1, 561·0, 555·7, 518·58, 516·5, 450·95, 420·10, 415·95 and
394·85. Altogether 80 lines have been observed in this spectrum and 119 in
the blue spectrum, of which 26 are common to both spectra.[9]
2. According to Rayleigh and Ramsay the solubility of argon in water
is approximately 4 volumes in 100 volumes of water at 13°. Thus argon
is nearly 2½ times more soluble than nitrogen, and its solubility approaches
that of oxygen. Direct experiment proves that nitrogen obtained
from air from boiled water is heavier than that obtained straight from the
atmosphere. This again is an indirect proof of the presence of argon in
air.
3. The ratio k of the two specific heats (at a constant pressure and at[496]
a constant volume) of argon was determined by Rayleigh and Ramsay by the
method of the velocity of sound (see Chapter XIV., Note 7 and Chapter VII.,
Note 26) and was found to be nearly 1·66, that is greater than for those gases
whose molecules contain two atoms (for instance, CO, H2, N2, air, &c., for
which k is nearly 1·4) or those whose molecules contain three atoms (for
instance, CO2, N2O, &c., for which k is about 1·3), but closely approximate
to the ratio of the specific heats of mercury vapour (Kundt and Warburg,
k = 1·67). And as the molecule of mercury vapour contains one atom, so it
may be said that argon is a simple gaseous body whose molecule contains
one atom.[10] A compound body should give a smaller ratio. The experiments
upon the liquefaction of argon, which we shall presently describe, speak
against the supposition that argon is a mixture of two gases. The importance
of the results in question makes one wish that the determinations of the
ratio of the specific heats (and other physical properties) might be confirmed
with all possible accuracy.[11] If we admit, as we are obliged to do for the
present, that argon is a new element, its density shows that its atomic weight
must be nearly 40, that is, near to that of K = 39 and Ca = 40, which does
not correspond to the existing data respecting the periodicity of the properties[497]
of the elements in dependence upon their atomic weights, for there is no
reason on the basis of existing data for admitting any intermediate elements
between Cl = 35·5 and K = 39, and all the positions above potassium in the
periodic system are occupied. This renders it very desirable that the velocity
of sound in argon should be re-determined.[12]
4. Argon was liquefied by Professor Olszewsky, who is well known for his
classical researches upon liquefied gases. These researches have an especial
interest since they show that argon exhibits a perfect constancy in its[498]
properties in the liquid and critical states, which almost[13] disposes of the supposition
that it contains a mixture of two or more unknown gases. As the
first experiments showed, argon remains a gas under a pressure of 100
atmospheres and at a temperature of -90°; this indicated that its critical
temperature was probably below this temperature, as was indeed found to
be the case when the temperature was lowered to -128°·6[14] by means of
liquid ethylene. At this temperature argon easily liquefies to a colourless
liquid under 38 atmospheres. The meniscus begins to disappear at between[499]
-119°·8 and -121°·6, mean -121° at a pressure of 50·6 atmospheres. The
vapour tension of liquid argon at -128°·6, is 38·0 atmospheres, at -187°
it is one atmosphere, and at -189°·6 it solidifies to a colourless substance
like ice. The specific gravity of liquid argon at about -187° is nearly 1·5,
which is far above that of other liquefied gases of very low absolute boiling
point.
The discovery of argon is one of the most remarkable chemical acquisitions
of recent times, and we trust that Lord Rayleigh and Professor Ramsay,
who made this wonderful discovery, will further elucidate the true nature of
argon, as this should widen the fundamental principles of chemistry, to which
the chemists of Great Britain have from early times made such valuable
contributions. It would be premature now to give any definite opinions
upon so new a subject. Only one thing can be said; argon is so inert that
its rôle in nature cannot be considerable, notwithstanding its presence in the
atmosphere. But as the atmosphere itself plays such a vast part in the
life of the surface of the earth, every addition to our knowledge of its composition
must directly or indirectly react upon the sum total of our knowledge
of nature.
[501]
INDEX OF AUTHORITIES
- Abasheff, i. 75
- Abel, ii. 56, 326, 410
- Acheson, ii. 107
- Adie, ii. 186
- Alexéeff, i. 75, 94
- Alluard, i. 458
- Amagat, i. 132, 135, 140
- Amat, ii. 171
- Ammermüller, i. 504
- Ampère, i. 309
- Andréeff, i. 251
- Andrews, i. 136, 203
- Angeli, i. 266
- Ansdell, i. 451
- Arfvedson, i. 575
- Arrhenius, i. 89, 92, 389
- Aschoff, ii. 313
- Askenasy, i. 508
- Aubel, ii. 45
- Aubin, i. 238
- Avdéeff, i. 618; ii. 484
- Avogadro, i. 309
- Babo, v., i. 93, 200, 203
- Bach, i. 394
- Bachmetieff, ii. 31
- Baeyer, v., i. 507
- Bagouski, i. 384
- Bailey, i. 449; ii. 29
- Baker, i. 318, 403
- Balard, i. 480, 494, 495, 505
- Ball, ii. 414
- Bannoff, i. 506
- Barfoed, ii. 53
- Baroni, i. 331
- Barreswill, ii. 282
- Baudrimont, ii. 35
- Baumé, i. 193
- Baumgauer, ii. 20
- Baumhauer, i. 495
- Bayer, ii. 76, 159
- Bazaroff, i. 409; ii. 24, 68, 486
- Becher, i. 17
- Becker, i. 16
- Beckmann, i. 91, 496; ii. 156
- Becquerel, i. 228; ii. 97, 220
- Beilby, i. 71
- Beilstein, i. 373; ii. 188
- Beketoff, i. 120, 122, 124, 146, 403, 459, 466, 534, 541, 574, 577; ii. 87, 102, 289, 429
- Bender, i. 476
- Benedict, ii. 65
- Berglund, ii. 229
- Bergman, i. 27, 435; ii. 100
- Berlin, i. 95
- Bernouilli, i. 81
- Bernthsen, ii. 228
- Bert, i. 86, 153
- Bertheim, ii. 337
- Berthelot, i. 171, 173, 189, 199, 229, 230, 258, 264, 266, 267, 272, 83, 289, 351, 372, 393, 394, 405, 415, 424, 438, 457, 463, 502, 506, 507, 518, 529, 537, 582; ii. 23, 57, 207, 209, 251, 253, 259, 345, 367
- Berthier, ii. 8
- Berthollet, i. 27, 31, 105, 433, 434, 459, 470, 502, 609
- Berzelius, i. 131, 148, 194, 255, 379; ii. 8, 100, 102, 147, 148, 219, 281, 300, 485
- Besson, i. 288; ii. 67, 70, 105, 179
- Beudant, ii. 7, 8
- Bineau, i. 100, 271, 452, 504; ii. 239
- Binget, i. 75
- Blaese, ii. 188
- Blagden, i. 91, 428
- Blake, ii. 30
- Blitz, ii. 184
- Blomstrand, ii. 299
- Boerwald, ii. 279
- Böttger, i. 595
- Bogorodsky, i. 574
- Boilleau, i. 415
- Boisbaudran, L. de, i. 97, 102, 572, 600; ii. 6, 26, 90, 82, 284, 483[502]
- Bornemann, i. 509
- Botkin, ii. 30
- Bouchardat, ii. 45
- Boullay, ii. 55
- Bourdiakoff, i. 584, 617
- Boussingault, i. 131, 157, 233, 235, 525, 615
- Boyle, i. 124
- Brand, ii. 150
- Brandau, i. 481
- Brandes, i. 72
- Bravais, i. 233
- Brauner, i. 490, 491; ii. 26, 59, 94, 96, 97, 134, 144, 194, 271, 483
- Brewster, i. 569
- Brigham, ii. 193
- Brodie, i. 212, 351, 405; ii. 252
- Brooke, ii. 357
- Brown, i. 81, 88
- Brugellmann, i. 616
- Brunn, ii. 182, 189
- Bruyn, i. 262
- Brühl, i. 263, 337
- Brunner, i. 124, 146, 263, 534; ii. 230, 309
- Buchner, i. 615
- Buckton, ii. 143
- Buff, ii. 103
- Bunge, i. 288
- Bunsen, i. 43, 69, 78, 117, 180, 465, 568, 575, 576, 577; ii. 27, 289
- Bussy, i. 75, 594, 619
- Butleroff, i. 143
- Bystrom, i. 585
- Cagniard de Latour, i. 135, 345
- Cahours, ii. 143, 173
- Cailletet, i. 132, 138; ii. 45
- Calderon, i. 596
- Callender, i. 134
- Calvert, i. 484; ii. 45
- Cannizzaro, i. 584, 587
- Carey-Lea, ii. 420, 424, 425, 432
- Carius, i. 69, 481
- Carnelley, i. 483, 515, 555; ii. 22, 29, 30, 31, 64, 143, 486
- Carnot, ii. 294, 361
- Caron, i. 595, 604, 610; ii. 336
- Carrara, i. 213
- Cass, ii. 85
- Castner, i. 431, 535, 541
- Cavazzi, ii. 160, 172, 182
- Cavendish, i. 113, 125, 228; ii. 493
- Chabrié, i. 229
- Chappuis, i. 50, 199, 205, 264
- Chapuy, i. 59
- Cheltzoff, i. 393, 457, 582; ii. 41, 247
- Cherikoff, ii. 102
- Chertel, ii. 245
- Chevillot, ii. 311
- Chevreul, i. 530
- Chigeffsky, ii. 62
- Christomanos, i. 511
- Chroustchoff, i. 353, 444; ii. 122
- Chydenius, ii. 148, 485
- Ciamician, i. 565, 573; ii. 486
- Clark, i. 26
- Classen, ii. 146
- Clausius, i. 81, 93, 140, 142, 212, 309, 491
- Clement, i. 494
- Clève, ii. 26, 94, 97, 484
- Cloez, i. 207, 246, 377
- Clowes, i. 242
- Collendar, i. 134
- Comaille, i. 596
- Comb, ii. 81
- Connell, i. 508
- Coppet, i. 91, 428, 601
- Corenwinder, i. 501
- Cornu, i. 565
- Courtois, i. 494
- Cracow, ii. 380
- Crafts, i. 380 ; ii. 80, 83
- Cremers, ii. 100
- Croissier, i. 251
- Crompton, i. 247
- Crookes, i. 229, 617; ii. 20, 91, 96, 440, 491
- Crum, ii. 79, 311
- Cundall, i. 611
- Curtius, i. 258, 265
- Dahl, ii. 59
- Dalton, i. 29, 78, 81, 109, 206, 271, 322
- Dana, ii. 8
- Davies, i. 484
- Davy, i. 37, 114, 195, 255, 364, 460, 463, 484, 489, 494, 533, 541, 594, 604, 617
- Deacon, i. 599
- Debray, i. 609; ii. 45, 122, 291, 293, 384, 385
- De Chancourtois, ii. 20, 26
- De Forcrand, ii. 106, 211
- De Haën, ii. 189
- De Heen, i. 140
- Delafontaine, ii. 97, 148, 198
- De la Rive, i. 198; ii. 226
- Del-Rio, ii. 197
- De Saussure, i. 235, 240
- De Schulten, ii. 48
- Deville, St.-Claire, i. 4, 36, 118, 143, 179, 180, 227, 239, 280, 281, 301, 320, 392, 393, 399, 459, 467, 476, 477, 500, 534, 595, 608, 609; ii. 48, 80, 83, 85, 102, 147, 156, 198, 289, 309, 321, 352, 373, 374, 429
- De Vries, i. 62, 64, 429[503]
- Dewar, i. 3, 5, 135, 139, 163, 298, 563, 565, 569, 585; ii. 176, 220
- Dick, ii. 414
- Dingwall, i. 486
- Ditte, i. 72, 403, 430, 457, 509, 539, 618; ii. 64, 65, 85, 189, 249
- Dittmar, i. 100, 452; ii. 240
- Divers, i. 274, 294; ii. 54
- Dixon, i. 171
- Döbereiner, i. 145
- Dokouchaeff, i. 344
- Donny, i. 534
- Dossios, i. 502
- Draper, i. 465
- Drawe, ii. 161
- Drebbel, i. 294
- Dulong, i. 131, 148, 437
- Dumas, i. 28, 131, 148, 150, 233, 302, 320, 379, 471, 476, 584, 586, 604; ii. 22, 37, 62, 101, 156, 420
- Dumont, ii. 197
- Ebelmann, ii. 65
- Eder, i. 566
- Edron, ii. 95
- Edwards, ii. 311
- Egoreff, i. 569
- Eissler, i. 553
- Elbers, ii. 221
- Emich, i. 286, 287
- Emilianoff, ii. 126
- Engel, i. 457; ii. 130, 132, 189, 206
- Engelhardt, i. 530
- Eötvös, i. 333
- Erdmann, i. 150
- Ernst, i. 399
- Eroféeff, i. 352
- Esson, ii. 314
- Étard, i. 72, 516, 615; ii. 288, 335, 356
- Ettinger, i. 53, 312
- Famintzin, i. 611
- Faraday, i. 134, 177, 296, 385, 463, 464
- Favorsky, i. 373
- Favre, i. 120, 172, 267, 582; ii. 83, 259, 284, 380
- Fick, i. 62
- Fisher, ii. 424
- Fizeau, ii. 31, 429
- Flavitzky, i. 21
- Fleitmann, ii. 170
- Foerster, ii. 375, 389
- Forchhammer, ii. 311
- Fordos, ii. 257
- Fortmann, ii. 230, 366
- Fourcroy, i. 114
- Fowler, i. 449
- Frank, ii. 88
- Franke, ii. 311, 313
- Frankel, ii. 294
- Frankenheim, ii. 7
- Frankland, i. 178, 357, 486; ii. 16, 143
- Fraunhofer, i. 563
- Frémy, i. 228, 489, 492; ii. 74, 131, 133, 142, 229, 290, 359
- Freyer, i. 171, 488
- Friedheim, ii. 197, 294
- Friedel, i. 353, 472; ii. 80, 83, 103, 122
- Friedrich, i. 49; ii. 144
- Fritzsche, i. 94, 285, 600, 612; ii. 125, 218, 280, 341
- Fromherz, ii. 313
- Fürst, i. 484
- Galileo, i. 7
- Garni, i. 582
- Garzarolli-Thurnlackh, i. 481
- Gattermann, i. 596; ii. 102, 104
- Gautier, i. 585
- Gavaloffsky, i. 160
- Gay-Lussac, i. 40, 61, 71, 93, 170, 302, 307, 406, 412, 460, 463, 464, 467, 500, 506, 508, 511, 515, 534, 539; ii. 8, 56, 256
- Geber, i. 17
- Gélis, ii. 257
- Genth, ii. 359
- Georgi, ii. 197
- Georgiewics, ii. 64
- Gerberts, i. 528
- Gerhardt, i. 196, 309, 357, 388
- Gerlach, i. 525
- Gernez, i. 97; ii. 205
- Geuther, i. 281, 283, 285; ii. 176
- Gibbs, i. 140, 464; ii. 293, 410
- Girault, i. 498
- Gladstone, i. 337, 438, 573; ii. 213
- Glatzel, ii. 213, 289, 309
- Glauber, i. 17, 26, 193, 432
- Glinka, i. 607
- Goldberg, i. 93
- Gooch, i. 484
- Gore, i. 489, 492, 493
- Graham, i. 62, 63, 98, 143, 155, 388, 429, 518, 601; ii. 77, 114, 131, 163, 170, 296, 307
- Granger, ii. 157, 410
- Grassi, i. 88
- Green, ii. 310
- Greshoff, i. 403
- Griffiths, i. 135
- Grimaldi, i. 537
- Groth, ii. 10[504]
- Grouven, i. 615
- Grove, i. 118, 119
- Grünwald, i. 573
- Grützner, ii. 296
- Guckelberger, ii. 84
- Guibourt, ii. 53
- Guldberg, i. 439, 464
- Güntz, i. 575; ii. 430
- Gustavson, i. 443, 444, 472, 505, 547; ii. 29, 175
- Guthrie, i. 99, 428, 601
- Guy, i. 136
- Habermann, ii. 210
- Hagebach, i. 573
- Hagen, i. 337
- Haitinger, i. 593
- Hammerl, i. 613
- Hanisch, ii. 233
- Hannay, i. 352; ii. 135
- Harcourt, ii. 314
- Hargreaves, i. 515
- Harris, ii. 52
- Hartley, i. 573; ii. 486
- Hartog, ii. 268
- Hasselberg, i. 566
- Haüy, ii. 7
- Haughton, ii. 20
- Häussermann, i. 483
- Hautefeuille, i. 199, 205, 264, 409, 414, 476, 477, 501, 538; ii. 102, 122, 379
- Hayter, ii. 175
- Hemilian, i. 132
- Hempel, i. 59, 524
- Henkoff, i. 530
- Henneberg, ii. 170
- Henning, ii. 3
- Henry, i. 78, 81
- Hérard, ii. 191
- Hermann, ii. 8, 47, 197
- Hermes, i. 529
- Hertz, ii. 156
- Hess, i. 178, 588
- Heycock, i. 537; ii. 128, 448
- Hillebrand, ii. 26, 93, 94, 484
- Hintze, ii. 10
- Hirtzel, ii. 55
- Hittorf, ii. 155
- Hodgkinson, ii. 432
- Höglund, ii. 94
- Hofmann, i. 302; ii. 146, 218, 447
- Holtzmann, i. 505
- Hoppé-Seyler, i. 611
- Horstmann, i. 408
- Houzeau, i. 202
- Hughes, ii. 212
- Hugo, ii. 21
- Humboldt, i. 170
- Humbly, i. 493; ii. 311
- Hutchinson, i. 491
- Huth, ii. 20
- Huyghens, i. 569
- Ikeda, ii. 152
- Ilosva, i. 202
- Inostrantzeff, i. 345; ii. 4
- Isambert, i. 250, 257, 408; ii. 41
- Ittner, i. 412
- Janssen, i. 569
- Jawein, ii. 170
- Jay, i. 258
- Jeannel, i. 104
- Joannis, i. 251, 255, 405, 537, 559
- Jörgensen, i. 498; ii. 359, 361, 376
- Johnson, ii. 45
- Jolly, i. 233
- Joly, ii. 384, 385
- Kamensky, ii. 414
- Kammerer, i. 286, 462, 509; ii. 297
- Kane, ii. 57
- Kapoustin, i. 403
- Karsten, i. 427, 428, 541, 599
- Kassner, i. 158
- Kayander, i. 133, 384; ii. 46
- Keiser, i. 150
- Kekulé, i. 358, 369, 507; ii. 294
- Keyser, ii. 33
- Khichinsky, i. 440
- Kimmins, i. 510
- Kirchhoff, i. 567
- Kirmann, ii. 268
- Kirpicheff, i. 132
- Kjeldahl, i. 249; ii. 249
- Klaproth, ii. 7, 145, 147, 301
- Kleiber, i. 570
- Klimenko, i. 465
- Klobb, ii. 357
- Klodt, i. 426
- Knopp, ii. 338
- Knox, i. 489
- Kobb, ii. 125
- Kobell, ii. 197
- Koch, i. 44
- Kohlrausch, i. 245, 525
- Kolbe, i. 506
- Kolotoff, i. 263
- Konovaloff, i. 39, 65, 90, 93, 100, 140, 142, 172, 322; ii. 235, 268
- Kopp, i. 586, 587, 612; ii. 3, 37
- Koucheroff, i. 373
- Kouriloff, i. 209, 247, 274; ii. 41
- Kournakoff, i. 393; ii. 294, 365, 396[505]
- Kraevitch, i. 133, 134
- Kraft, i. 65, 88, 537
- Krafts, i. 393
- Kreisler, i. 233
- Kremers, i. 87, 443; ii. 244, 427
- Kreider, i. 484
- Krönig, i. 81
- Krüger, ii. 282, 284
- Krüss, ii. 355, 442, 447, 486
- Kubierschky, ii. 213
- Kühlmann, i. 608
- Kuhnheim, i. 612
- Kundt, i. 328, 589; ii. 496
- Kvasnik, ii. 57
- Kynaston, i. 522
- Lachinoff, i. 116, 457; ii. 410
- Ladenburg, ii, 103
- Lamy, ii. 91
- Landolt, i. 7, 337
- Lang, i. 399
- Langer, i. 226, 459, 462
- Langlois, i. 570 ; ii. 257
- Latchinoff (see Lachinoff), i. 103, 352
- Laurent, i. 28, 196, 388, 471, 526; ii. 7, 9, 10, 117, 292
- Laurie, i. 106; ii. 32, 442, 486
- Lavenig, i. 140
- Lavoisier, i. 7, 29, 49, 114, 131, 155, 379, 459
- Leblanc, ii. 8
- Le Chatelier, i. 158, 172, 350, 393, 399, 585, 588, 611; ii. 51, 65, 420
- Le Duc, i. 131, 170
- Lémery, i. 125
- Lemoine, i. 501; ii. 155
- Lerch, i. 405
- Leroy, i. 285
- Lescœur, i. 103
- Leton, ii. 425
- Levy, ii. 102
- Lewes, i. 371
- Lewy, i. 232
- Lidoff, ii. 209
- Liebig, i. 195, 388, 495, 527; ii. 56
- Linder, ii. 223
- Liés-Bodart, i. 604, 612
- Lisenko, i. 373
- Liveing, i. 563, 569
- Lockyer, i. 565, 569
- Loew, ii. 376
- Löwel, i. 525, 600; ii. 45, 284, 286
- Loewig, i. 528; ii. 77
- Loewitz, i. 96
- Lossen, i. 262
- Louget, i. 489
- Louguinine, i. 360
- Louise, ii. 81
- Lovel, i. 515; ii. 338
- Lubavin, i. 593
- Lubbert, ii. 85, 170
- Ludwig, i. 463
- Luedeking, ii. 194
- Luff, ii. 321
- Lunge, ii. 244, 246
- Lüpke, ii. 157
- Lvoff, i. 358
- Maack, i. 596
- Mac Cobb, i. 612
- Mac Laurin, i. 553
- McLeod, ii. 180
- Magnus, i. 93, 510
- Mailfert, i. 199
- Malaguti, i. 437; ii. 300
- Mallard, i. 172, 393, 588; ii. 4
- Mallet, i. 493
- Maquenne, i. 349, 620, 621
- Marchand, i. 150
- Marchetti, ii. 288
- Maresca, i. 534
- Marguerite, ii. 292
- Marignac, i. 198, 199, 233, 428, 430, 453, 454, 518, 525, 600, 601; ii. 6, 9, 95, 101, 194, 197, 198, 234, 239, 241, 244, 292, 293, 295, 357, 440, 486
- Markleffsky, i. 273
- Markovnikoff, i. 373
- Maroffsky, ii. 138
- Marshall, ii. 253, 365
- Matigon, i. 258, 266
- Maumené, i. 258
- Maxwell, i. 81
- Mayow, i. 17
- Mendeléeff, i. 99, 132, 133, 136, 141, 275, 321, 357, 373, 377, 406, 426, 427, 428, 506, 587, 596; ii. 27, 33, 93, 94
- Menschutkin, i. 171
- Mente, ii. 270
- Mermé, i. 462
- Merz, i. 505
- Meselan, i. 463
- Metzner, ii. 189
- Metchikoff, i. 44
- Meusnier, i. 114
- Meyer (Lothar), i. 226, 321, 403; ii. 21, 24, 26, 29, 33, 486
- Meyer (Victor), i. 135, 171, 294, 303, 320, 427, 459, 462, 467, 488, 506, 508, 558; ii. 43, 48, 52, 80, 129, 184
- Meyerhoffer, ii. 410
- Miasnikoff, i. 372
- Michaelis, ii. 175
- Michel, i. 65, 88
- Millon, i. 481, 484, 508
- Mills, ii. 20[506]
- Mitchell, i. 156
- Mitscherlich, i. 428, 527; ii. 1, 5, 8, 156, 184, 311, 313
- Moissan, i. 202, 349, 353, 490, 564, 585, 621; ii. 66, 67, 70, 88, 100, 107, 147, 174, 196, 289, 295, 309, 311, 313, 321
- Mond, i. 129, 400, 405; ii. 345, 367
- Monge, i. 114
- Monnier, i. 611
- Montemartini, i. 279
- Moraht, ii. 384
- Moreau, ii. 298
- Morel, i. 549
- Mosander, ii. 97
- Mühlhäuser, ii. 66, 107
- Muir, ii. 193
- Mulder, i. 515
- Müller-Erzbach, i. 103
- Müller, i. 427; ii. 425
- Munster, ii. 443
- Müntz, i. 238, 241, 420, 553
- Muthmann, ii. 273
- Mylius, ii. 375, 389
- Naschold, i. 483
- Nasini, i. 496; ii. 156
- Natanson, i. 282, 409
- Natterer, i. 132, 135, 141, 385
- Naumann, i. 399, 408
- Nernst, i. 62, 148; ii. 3, 50
- Nensky, i. 245
- Neville, i. 537; ii. 128, 448
- Newlands, ii. 21, 26
- Newth, i. 505
- Newton, i. 7, 29
- Nicklès, ii. 10
- Nikolukin, i. 491; ii. 144
- Nilson, i. 618; ii. 26, 37, 80, 83, 91, 94, 95, 271, 378, 483
- Nordenskiöld, i. 241
- Norton, i. 76; ii. 94
- Nuricsán, ii. 264
- Odling, ii. 52
- Offer, i. 99
- Ogier, i. 321, 509; ii. 159, 182
- Olszewski, i. 139, 569; ii. 491, 497
- Oppenheim, i. 506
- Ordway, ii. 80
- Osmond, ii. 326
- Ossovetsky, ii. 137
- Ostwald, i. 89, 92, 389, 441, 443
- Oumoff, i. 62
- Pallard, i. 491; ii. 83
- Panfeloff, i. 603
- Paracelsus, i. 17, 125, 129, 379
- Parkinson, i. 596,
- Pashkoffsky, i. 595
- Pasteur, i. 44, 241, 242
- Paterno, i. 496; ii. 156
- Pattison Muir, i. 436
- Pebal, i. 315, 484
- Péchard, ii. 282, 294, 296, 297
- Pekatoros, i. 465
- Peligot, ii. 299, 301
- Pelopidas, ii. 22, 481
- Pelouze, i. 463, 464, 480, 610; ii. 229
- Penfield, i. 545; ii. 370
- Perkin, i. 558; ii. 244
- Perman, i. 537
- Personne, i. 75, 506, 537
- Petit, i. 584, 586
- Petrieff, i. 440
- Pettenkofer, ii. 22
- Pettersson, i. 618, 619; ii. 37, 80, 83, 91, 197, 484
- Pfaundler, i. 445; ii. 241, 430
- Pfeiffer, i. 64
- Pfordten, V. der, ii. 420
- Phipson, i. 596; ii. 59
- Piccini, ii. 23, 146, 197, 288, 298
- Pici, ii. 57
- Pickering, i. 88, 91, 99, 104, 106, 272, 333, 452, 517, 525, 529, 613; ii. 241, 245, 246, 247
- Pictet, i. 81, 129, 137; ii. 31, 241
- Picton, ii. 223
- Pierre, i. 452, 495; ii. 226, 485
- Pierson, i. 93
- Pigeon, ii. 377
- Pionchon, i. 585
- Pistor, i. 399
- Plantamour, ii. 5
- Plaset, ii. 289
- Plessy, ii. 257
- Plücker, i. 572
- Poggiale, i. 427
- Poiseuille, i. 355
- Poleck, ii. 296
- Poluta, ii. 30
- Popp, ii. 232
- Potilitzin, i. 96, 97, 98, 445, 486, 499, 502, 509, 612; ii. 29, 357
- Pott, ii. 100
- Poulenc, ii. 174, 289
- Prange, ii. 422
- Prelinger, ii. 310
- Priestley, i. 17, 154, 159, 298, 379, 402
- Pringsheim, i. 465
- Prost, i. 98, 486
- Prout, i. 31; ii. 439
- Puchot, i. 452
- Pullinger, ii. 389
- Quincke, i. 427, 495[507]
- Rammelsberg, i. 430, 510, 525; ii. 26, 161, 485
- Ramsay, i. 133, 140, 141, 232, 247, 333, 495, 496, 581; ii. 128, 491
- Rantsheff, ii. 20
- Raoult, i. 91, 274, 330, 331, 332, 429
- Rascher, ii. 85
- Raschig, i. 263; ii. 229
- Rathke, i. 399
- Ray, i. 17
- Rayleigh, i. 226, 232, 491
- Rebs, ii. 213, 217
- Recoura, i. 332; ii. 289
- Regnault, i. 40, 53, 54, 90, 93, 131, 133, 298, 443, 495, 584, 587, 588; ii. 50, 208, 238
- Reich, ii. 91
- Reiset, i. 238
- Remsen, ii. 335
- Retgers, ii. 157, 158, 180
- Reychler, ii. 65
- Reynolds, i. 581
- Richards, i. 526, 585; ii. 32, 432
- Riche, i. 509; ii. 127, 292
- Richter, i. 193, 194; ii. 91
- Ridberg, ii. 21, 24, 486
- Riddle, i. 135
- Rideal, ii. 297
- Roberts-Austen, ii. 486
- Robinson, i. 515
- Rodger, ii. 213, 263
- Rodwell, i. 17
- Roebuck, i. 294
- Röggs, ii. 119
- Rohrer, ii. 343
- Roozeboom, i. 106, 452, 453, 464, 496, 506, 511, 599, 613; ii. 3, 226, 341, 410
- Roscoe, i. 80, 100, 101, 379, 452, 463, 485, 486, 568, 572; ii. 26, 194, 196, 197, 297, 303, 485
- Rose, i. 436, 437, 518, 525, 608, 612; ii. 8, 230, 235, 248, 281, 363, 428, 485
- Rosenberg, ii. 351
- Rossetti, i. 428
- Rouart, Le, ii. 86
- Rousseau, i. 354; ii. 337, 366, 378
- Roux, ii. 81
- Rudberg, ii. 136
- Rücker, i. 142
- Rüdorff, i. 91, 428, 598, 601
- Rybalkin, i. 455
- Sabanéeff, i. 371
- Sabatier, i. 284; ii. 66
- Saint Edmé, ii. 335
- Saint Gilles, i. 431
- Sakurai, i. 331
- Salzer, ii. 161
- Sarasin, ii. 122
- Sarrau, i. 140, 142
- Saunders, ii. 189
- Scharples, i. 576
- Scheele, i. 155, 161, 412, 459, 462, 608; ii. 100, 150, 291
- Scheffer, i. 453
- Scheibler, ii. 292, 296
- Scherer, ii. 8
- Schiaparelli, ii. 318
- Schidloffsky, i. 238
- Schiloff, i. 212
- Schlamp, i. 332
- Schiff, i. 430, 588; ii. 106, 267
- Schloesing, i. 238, 239, 240, 553, 610
- Schmidt, i. 539
- Schneider, i. 89
- Schöne, i. 208, 209, 211, 394, 617; ii. 15, 72, 219, 251, 488
- Schönebein, i. 198, 202, 208, 212, 509; ii. 228, 463
- Schottländer, ii. 447
- Schröder, i. 75
- Schroederer, ii. 366
- Schrötter, ii. 153, 284
- Schützenberger, i. 511, 579; ii. 102, 107, 228, 367, 389
- Schuliachenko, i. 608
- Schuller, ii. 180
- Schultz, i. 518; ii. 273
- Schulze, i. 98; ii. 215
- Schuster, i. 572
- Schwicker, ii. 227, 230
- Scott, i. 405, 537, 558
- Sechenoff, i. 80, 86
- Seelheim, ii. 379
- Sefström, ii. 197
- Selivanoff, i. 476, 507, 508
- Senderens, i. 284
- Serullas, i. 485
- Setterberg, i. 576
- Seubert, ii. 27, 83, 343, 442
- Sewitsch, i. 372
- Shaffgotsch, i. 555
- Shapleigh, ii. 95
- Shenstone, i. 611
- Shields, i. 333
- Shishkoff, i. 276; ii. 56
- Silberman, i. 120, 172; ii. 259
- Sims, ii. 268
- Skraup, ii. 346
- Smith, i. 271
- Smithson, ii. 100
- Snyders, ii. 100
- Sokoloff, ii. 85, 122
- Solet, i. 509
- Sonstadt, ii. 443
- Sorby, i. 88[508]
- Soret, i. 66, 202, 203, 427
- Spring, i. 38, 98, 434, 486; ii. 45, 50, 133, 223, 258, 288, 314, 423, 427
- Stadion, i. 485
- Stahl, i. 16
- Stas, i. 7, 233, 379, 428, 498, 581; ii. 420, 434, 485
- Staudenmaier, ii. 168
- Stcherbakoff, i. 97, 428, 458, 601
- Stohmann, i. 359, 360, 396
- Stokes, i. 355
- Stortenbeker, i. 511
- Stromeyer, ii. 47
- Struvé, i. 208, 612
- Tait, i. 203
- Tammann, i. 91, 148; ii. 170, 247
- Tanatar, i. 511
- Tchitchérin, ii. 21
- Terreil, ii. 313
- Than, i. 317
- Thénard, i. 207, 229, 460, 464, 534, 539; ii. 251
- Thillot, ii. 170
- Thilorier, i. 385
- Thomsen, i. 111, 120, 124, 131, 173, 189, 267, 359, 389, 396, 441, 453, 466, 472, 494, 502, 515, 529, 555, 582; ii. 9, 32, 50, 55, 105, 165, 208, 224, 264, 368, 370, 438, 442
- Thorpe, i. 142, 285, 445, 493; ii. 27, 160, 173, 213, 259, 263, 268, 301, 313, 442, 486
- Thoune, i. 294, 295
- Tiemman, i. 213
- Tilden, i. 516
- Timeraséeff, i. 170
- Timoféeff, i. 78
- Tessié du Motay, i. 158
- Tissandier, i. 78
- Titherley, i. 539
- Tivoli, ii. 183
- Tomassi, ii. 339
- Topsöe, i. 506
- Tourbaba, i. 88; ii. 247
- Trapp, i. 511
- Traubé, i. 312, 611; ii. 270
- Troost, i. 64, 274, 281, 320, 409, 414, 500, 538; ii. 80, 83, 102, 147, 156, 254, 379
- Tscherbacheff, i. 577
- Tutton, i. 543; ii. 160, 174, 412
- Umoff, i. 429
- Unverdorben, ii. 280
- Urlaub, ii. 301
- Valentine, i. 17
- Van der Heyd, i. 599
- Van der Plaats, i. 496; ii. 438
- Van der Waals, i. 82, 140
- Van Deventer, i. 599
- Van Helmont, i. 379
- Van Marum, i. 198
- Van't Hoff, i. 64, 65, 331, 599; ii. 3
- Vare, ii. 55
- Vauquelin, i. 114, 619; ii. 7
- Veeren, i. 612; ii. 45
- Veley, i. 279
- Verneuille, ii. 225
- Vernon, ii. 151
- Vèzes, ii. 391
- Viard, ii. 285
- Vignon, ii. 126, 131
- Villard, i. 106, 296, 298
- Villiers, ii. 259
- Violette, i. 342, 345
- Violle, i. 301
- Vogt, i. 611
- Volkovitch, ii. 201
- Voskresensky, i. 345
- Waage, i. 439
- Wachter, i. 508
- Wagner, i. 357
- Wahl, ii. 310
- Walden, ii. 57
- Walker, ii. 143
- Walmer, i. 573
- Walter, ii. 256
- Walters, ii. 234
- Wanklyn, i. 100, 539
- Warburg, i. 589; ii. 496
- Warder, i. 450
- Warren, ii. 102
- Watson, i. 527; ii. 169
- Watts, i. 526
- Weber, i. 280, 583; ii. 83, 129, 131, 186, 230, 233, 234, 249
- Weith, i. 502
- Weitz, ii. 57
- Welch, ii. 425
- Weller, ii. 146
- Wells, i. 477, 545; ii. 57, 370
- Welsbach, ii. 96, 97
- Weltzien, i. 204, 595
- Wenzel, i. 193
- Weruboff (see Wyruboff), ii. 4
- Weselski, i. 507
- Weyl, i. 255
- Wheeler, i. 545
- Wichelhaus, ii. 179
- Wiedemann, i. 439, 588
- Wilhelmj, ii. 315
- Willgerodt, i. 508; ii. 29
- Williamson, ii. 268
- Wilm, ii. 376, 388[509]
- Winkler, i. 78, 79, 577, 594, 621; ii. 25, 30, 66, 97, 102, 124, 125, 147, 234, 246, 355, 483
- Wischin, ii. 384
- Wislicenus, i. 267, 294
- Witt, ii. 3
- Wöhler, i. 410, 619; ii. 85, 103, 107, 146, 285, 289, 420, 425
- Wollaston, i. 8
- Wreden, i. 507
- Wright, ii. 321
- Wroblewski, i. 79, 80, 106, 139, 387; ii. 226
- Wülfing, ii. 119
- Wülner, i. 91, 572
- Würtz, i. 301, 476; ii. 171, 173, 213, 267
- Wyruboff, ii. 4, 9
- Young, i. 134, 136, 140, 141, 247, 495, 496
- Zaboudsky, i. 354
- Zaencheffsky, i. 140
- Zimmermann, ii. 26, 303, 355, 485
- Zinin, i. 276
- Zörensen, i. 284
- Zorn, i. 295
[510]
SUBJECT INDEX
- Acid, acetic sp. gr. of solutions of, i. 59
- — arsenic, ii. 181
- — bismuthic, ii. 190
- — boric, ii. 64
- — carbamic, i. 408
- — chamber, i. 294
- — chloric, i. 482
- — chloroplatino-phosphorous, ii. 390
- — chlorosulphonic, ii. 268
- — chlorous, i. 481
- — chromic, i. 208; ii. 282
- — chromo-sulphuric, ii. 288
- — cyanic, i. 409
- — cyanuric, i. 409
- — dithionic, ii. 256
- — ferric, ii. 344
- — fluoboric, ii. 69
- — graphitic, i. 351
- — hydriodic, i. 501, 503, 505, 506
- — hydroborofluoric, ii. 69
- — hydrobromic, i. 80, 503, 505, 506
- — hydrochloric, i. 448, 451, 453
- — hydrocyanic, i. 406, 411
- — hydroferrocyanic, ii. 348
- — hydrofluoric, i. 49
- — hydrofluosilic, ii. 106
- — hydroplatinocyanic, ii. 386
- — hydrosulphurous, ii. 228
- — hydroruthenocyanic, ii. 388
- — hypochlorous, i. 479, 481
- — hyponitrous, i. 265, 294
- — hypophosphoric, ii. 161
- — hypophosphorous, ii. 172
- — iodic, i. 100, 508
- — isethionic, ii. 250
- — metantimonic, ii. 188
- — metaphosphoric, ii. 162, 169
- — metastannic, ii. 131
- — molybdic, ii. 292
- — nitric, i. 268, 272
- — Nordhausen, ii. 233
- — orthophosphoric, ii. 162
- — osmic, ii. 384
- — pentathionic, ii. 257
- Acid, percarbonic, i. 394
- — perchloric, i. 484
- — periodic, i. 510
- — permanganic, ii. 313
- — permolybdic, ii. 297
- — pernitric, i. 264
- — persulphuric, ii. 251
- — pertungstic, ii. 297
- — phosphamic, ii. 179
- — phosphamolybdic, ii. 293
- — phosphorous, ii. 171
- — polysilicic, ii. 117
- — pyrophosphoric, ii. 169
- — pyrosulphuric, ii. 234
- — silenic, ii. 272
- — silicotungstic, ii. 295
- — stannic, ii. 130
- — sulphonic, ii. 249
- — sulphuric, i. 76, 77, 89, 111, 290, 294; ii. 235, 238, 241
- — telluric, ii. 272
- — tetrathionic, ii. 257
- — thiocarbonic, ii. 263
- — thiocyanic, ii. 263
- — thionic, ii. 255
- — thiosulphuric, ii. 230
- — trithionic, ii. 257
- — tungstic, ii. 292, 294
- — vanadic, ii. 196
- Acids, i. 185
- — avidity of, i. 389, 442
- — basicity of, i. 387
- — complex, i. 197; ii. 293
- — fuming, i. 102
- — organic, i. 394, 396, 405
- Acetylene, i. 372
- Actinium, ii. 59
- Affinity, chemical, i. 26, 389
- Air, i. 131, 231, 233
- Alchemy, i. 14
- Alcohol, i. 53, 88
- Alkali, metals, i. 558, 577
- — waste, ii. 204
- Alkalis, i. 186[511]
- Allotropism, i. 207
- Alloys, i. 537; ii. 128
- Alumina, ii. 75
- Aluminium, ii. 70, 85
- — bromide, ii. 84
- — bronze, ii. 88
- — carbide, ii. 88
- — chloride, ii. 80, 83
- — double chlorides, ii. 84
- — fluoride, ii. 83
- — hydroxide, ii. 75
- — iodide, ii. 85
- — nitrate, ii. 80
- — sulphate, ii. 82
- Alums, ii. 5, 82, 343
- Alunite, ii. 80
- Amalgams, ii. 58
- Amides, i. 258, 406
- Amidogen, i. 258
- — hydrate, i. 258
- Amines, i. 416
- Ammonia, i. 229, 246
- — of crystallisation, i. 257
- — heat of solution of, i. 74
- — in air, i. 240
- — liquefaction of, i. 250
- — salts, i. 254
- — soda process, i. 524
- — solutions of, i. 80, 252
- Ammonium, i. 254
- — amalgam, i. 255
- — bicarbonate, i. 527
- — carbamate, i. 407, 408
- — carbonate, i. 407
- — cobalt salts, ii. 359
- — dichromate, ii. 279
- — molybdate, ii. 292
- — nitrate, i. 273, 274
- — nitrite, i. 284
- — phosphates, ii. 167
- — sulphate, ii. 269
- — sulphide, ii. 218
- Analogy of elements, i. 573, 578
- Anthracite, i. 345
- Antimoniuretted hydrogen, ii. 189
- Antimony, ii. 186
- — chlorides, ii. 189
- — oxides, ii. 187, 188
- — sulphides, ii. 221
- Aqua Regia, i. 467
- Aqueous radicle, i. 213
- Argon, i. 226, 232; App. II.I
- Arsenic, ii. 179
- — anhydride, ii. 181
- — sulphides, ii. 221
- — tribromide, ii. 181
- — trichloride, ii. 180
- — trifluoride, ii. 181
- Arsenious anhydride, ii. 184
- Arsenious oxychloride, ii. 180
- Arsenites, ii. 185
- Arseniuretted hydrogen, ii. 182
- Astrakhanite, i. 59
- Atmolysis, i. 156
- Atomic theory, i. 216
- — volumes, ii. 33
- — weights, i. 21
- Atoms and molecules, i. 322
- Barium, i. 614, 617
- — chlorate, i. 483
- — chloride, i. 615
- — hydroxide, i. 616
- — metatungstate, ii. 295
- — nitrate, i. 615
- — oxide, i. 616
- — peroxide, i. 157, 160, 209, 617
- — sulphate, i. 614, 615
- Bauxite, ii. 76
- Benzalazine, i. 258
- Berthollet's doctrine, i. 433
- Beryllium, i. 618
- — atomic weight of, i. 325, 618
- — chloride, i. 584
- — oxide, i. 619
- Binary theory, i. 195
- Bismuth, ii. 189
- — nitrates, ii. 192
- — oxides, ii. 190, 191
- Blast furnace, ii. 324
- Bleaching, i. 469
- — powder, i. 162, 477
- Boiling point, absolute, i. 130
- Borates, ii. 63
- Borax, ii. 61
- Boric anhydride, ii. 64
- Boron, ii. 60, 66
- — chloride, ii. 69
- — fluoride, ii. 67, 68
- — iodide, ii. 70
- — nitride, i. 227; ii. 67
- — oxide, ii. 60
- — specific heat of, i. 585
- — sulphide, ii. 62
- Bromides, ii. 32
- Bromine, i. 494
- Bronze, ii. 127
- Butyl alcohol, solubility of, i. 75
- Cadmium, ii. 47
- — iodide, ii. 48
- — oxide, ii. 48
- — sulphide, ii. 47
- Cæsium, i. 576
- Calcium, i. 590, 604
- — carbonate, i. 592, 608, 609, 610[512]
- Calcium chloride, i. 237, 612
- — — crystallohydrates of, i. 613
- — fluoride, i. 491
- — hypochlorite, i. 162
- — iodide, i. 604
- — peroxide, i. 607
- — phosphate, ii. 167
- — sulphate, i. 611
- — sulphide, ii. 220
- Calomel, ii. 54
- Carbamide, i. 409
- Carbides, i. 349, 353
- Carbon, i. 338
- — bisulphide, ii. 258
- — molecule of, i. 354
- — oxysulphide, ii. 264
- — tetrachloride, i. 473
- Carbonic anhydride, i. 379
- — — assimilation of by plants, i. 393
- — — dissociation of, i. 392, 393, 399
- — — in air, i. 238, 242
- — — liquid, i. 385
- — — solutions of, i. 80, 86
- — — specific heat of, i. 393
- Carbonic oxide, i. 396
- — — and nickel, i. 405
- Carborundum, ii. 107
- Carboxyl, i. 395
- Carnallite, i. 421, 544, 560
- Catalytic phenomena, i. 211
- Caustic potash, i. 550
- — soda, i. 529
- Cements, ii. 122
- Cerite metals, ii. 93
- Cerium, ii. 93
- Chamber crystals, i. 290; ii. 230
- Charcoal, i. 343
- Chemical change, rate of, ii. 314
- — transformations, i. 3
- Chloranhydrides, i. 468; ii. 174, 175, 177
- Chlorates, i. 482
- Chlorides, i. 455, 466; ii. 31
- Chlorine, i. 463
- — compounds, heat of formation of, i. 44
- — crystallohydrates of, i. 464
- — oxides, i. 479
- — preparation of, i. 460
- — solubility of, i. 463
- Chloroform, i. 473
- Chlorophosphamide, ii. 179
- Chloryl compounds, i. 476
- Chrome alum, ii. 283
- Chromic acid, i. 208
- — anhydride, ii. 280
- — oxide, ii. 284, 285
- Chromium, ii. 276, 289
- — chlorides, ii. 285
- — fluorides, ii. 280, 289
- Chromyl chloride, ii. 281
- Chryseone, ii. 108
- Clay, ii. 70
- Coal, i. 345
- Cobalt, ii. 353
- — dioxide, ii. 366
- — fluoride, ii. 358
- Cobaltamine salts, ii. 359
- Cobaltic oxide, ii. 362
- Cobalto-amine, ii. 359
- Cobaltous hydroxide, ii. 358
- Cohesion of liquids, i. 52
- Coke, i. 345
- Collodion cotton, i. 275
- Colloids, i. 63; ii. 77, 423
- Combination, chemical, i. 3
- Combining weights, i. 21; ii. 439
- Combustion, imperfect, i. 341
- — heat of, i. 172, 176, 399, 400
- Compounds, definite and indefinite, i. 31
- — types of, ii. 10
- Compressibility of solutions, i. 88
- Conductivity, electro-molecular, i. 389
- Contact reactions, i. 163, 290
- Copper, ii. 400
- — carbonate, ii. 411
- — complex salts of, ii. 412
- — nitrate, ii. 411
- — nitride, ii. 409
- — sulphate, ii. 413
- Corundum, ii. 75
- Critical points, i. 141
- Cryohydrates, i. 99
- Cryoscopic investigations of solutions, i. 90, 332
- Crystals, i. 51
- Crystalline form, ii. 7
- Crystallo-hydrates, i. 102
- Crystalloids, i. 63
- Cupellation, ii. 417
- Cyanides, i. 406
- Cyanogen, i. 406, 414
- — chloride, ii. 176
- Decomposition, chemical, i. 4
- Deliquescence, i. 104
- Delta metal, ii. 414
- Desiccator, i. 58
- Detonating gas, i. 115, 170, 173
- Depression of freezing point of solutions, i. 90, 92, 330
- Dialysis, i. 63; ii. 114
- Diamond, i. 350, 353
- Didymium, ii. 93
- Diffusion, rate of, i. 63
- Dimorphism, i. 610, ii. 178
- Disinfectants, i. 245
- Disodium orthophosphate, ii. 166[513]
- Dissociation, i. 36, 282, 608
- Distillation, dry, i. 4, 247, 342
- Dust, atmospheric, i. 241
- Efflorescence, i. 103
- Ekacadmium, ii. 59
- Ekasilicon, ii. 25
- Electro-chemical theory, i. 195
- Electric energy and thermal units, i. 582
- Electrolysis, i. 116
- Elements, i. 20
- — grouping of, ii. 1
- — typical, ii. 19
- Emulsions, i. 98
- Energy, chemical, i. 29
- Equations, chemical, i. 278
- Equivalents, law of, i. 194
- Equivalent weights, i. 581
- Ethane, i. 366
- Ether, critical points of, i. 141
- Ethylene, i. 370
- Ethyl silicates, i. 104
- Euchlorine, i. 484
- Eudiometer, i. 169
- Expansion, linear, of elements, ii. 31
- Explosion, rate of transmission of, i. 171
- Explosives, i. 275, 276
- Felspar, ii. 122
- Fermentation, i. 242
- Ferric chloride, i. 558; ii. 340
- — hydrates, ii. 339
- — nitrate, ii. 340
- — orthophosphate, ii. 342
- — oxide, ii. 339
- Ferrous chloride, ii. 335
- — sulphate, ii. 335
- — — solubility of, i. 72
- — sulphide, ii. 210
- Flame, i. 177, 179
- Fluoborates, ii. 69
- Fluorides, i. 491, 493
- Fluorine, i. 203, 489
- Fluorspar, i. 491
- Formula, chemical, i. 151, 326
- Freezing mixtures, i. 76
- Fuel, calorific capacity of, i. 360
- Furnace, electrical, i. 352
- Fusco-cobaltic salts, ii. 360
- Gadolinite metals, ii. 93
- Gallium, ii. 88, 90
- Gas, illuminating, i. 361
- — producers, i. 397
- Gases, absorption of, i. 348
- — diffusion of, i. 83
- — expansion of, i. 133
- — liquefaction of, i. 134, 135, 137
- — measurement of, i. 78, 300
- — solution of, i. 68, 78, 86
- — theory of, i. 81, 83, 140
- Germanium, ii. 26, 124
- — chloride, ii. 125
- — oxide, ii. 125
- Glass, i. 123
- — soluble, ii. 110
- Glauber's salt, i. 517
- Glycols, ii. 117
- Gold, ii. 442
- — alloys, i. 446, 447
- — chlorides, ii. 448, 450
- — colloid, ii. 447
- — cyanide, ii. 450
- — extraction of, ii. 444, 445
- — fulminating, ii. 450
- — oxides, ii. 448
- — refining, ii. 446
- Graduators, i. 424
- Graphite, i. 350, 351
- Gros' salt, ii. 393
- Guignet's green, ii. 285
- Gunpowder, i. 557
- Gypsum, i. 593, 611
- Halogens, i. 445, 487, 499
- Halogen compounds, heat of formation of, i. 494, 502; ii. 32
- — — boiling-points of, i. 502
- Hausmannite, ii. 10
- Helium, i. 570; ii. 498
- Hemimorphism, ii. 9
- Homeomorphism, ii. 8
- Homologous compounds, i. 358
- Humus, i. 344
- Hydrates, i. 109, 185
- Hydrazine, i. 258
- Hydrides, i. 621; ii. 23
- Hydrocarbons, i. 355, 359
- Hydrogen, i. 123, 129, 130, 142, 143, 146
- — pentasulphide, ii. 217
- — peroxide, i. 207, 312
- Hydrosols, i. 98
- Hydroxyl, i. 192, 213
- Hydroxylamine, i. 262
- Hypochlorites, i. 481
- Hyponitrites, i. 294
- Imides, i. 258
- Indium, ii. 27, 37, 88, 97
- Iodates, i. 509
- Iodides, ii. 32
- — of nitrogen, i. 507[514]
- Iodine, i. 320, 321, 496, 497, 498
- — chlorides of, i. 511
- Iodosobenzol, i. 508
- Iridious oxide, ii. 382
- Iridium, ii. 382
- Iron, i. 585; ii. 317, 322
- — and carbonic oxide, ii. 345
- — cast, ii. 325
- — nitride, ii. 346
- — ores, ii. 319
- — sulphate, ii. 335
- Isethionic acid, ii. 250
- Isomorphism, i. 203, 368; ii. 1, 4, 8
- Kaolin, ii. 70
- Lakes, ii. 77
- Lanthanum, ii. 93
- Laughing gas, ii. 297
- Law of Avogadro-Gerhardt, i. 309
- — — Berthollet, i. 445
- — — Boyle and Mariotte, i. 132
- — — combining weights, i. 221
- — — Dulong and Petit, i. 584
- — — equivalents, i. 194
- — — even numbers, i. 357
- — — Gay Lussac, i. 133, 304, 307
- — — Guldberg and Waage, i. 441
- — — Henry and Dalton, i. 78
- — — indestructibility of matter, i. 6
- — — Kirchoff, i. 568
- — — limits, i. 357
- — — maximum work, i. 120
- — — multiple proportions, i. 109, 214
- — — partial pressures, i. 82
- — — periodic, ii. 17
- — — phases, ii. 410
- — — reversed spectra, i. 568
- — — specific heats, i. 584
- — — substitution, i. 260, 365
- — — volumes, i. 304
- Lead, ii. 134
- — acetate, ii. 137
- — carbonate, ii. 140
- — chloride, ii. 139
- — chromate, ii. 136, 279
- — dioxide, ii. 142
- — nitrate, ii. 139
- — oxide, ii. 137
- — red, ii. 142
- — salts of, i. 491
- — tetrachloride, ii. 144
- — tetrafluoride, ii. 144
- — white, ii. 140
- Leucone, ii. 107
- Levigation, ii. 72
- Light, chemical action of, i. 465
- Lime, i. 605
- Liquids, boiling points of, i. 135
- Lithium, i. 574
- — carbonate, i. 575
- Litharge, ii. 137
- Litmus, i. 185
- Lixiviation, methodical, i. 521
- Luteocobaltic salts, ii. 359
- Magnus' salt, ii. 392
- Magnesia, i. 597
- Magnesium, i. 590, 594
- — carbonate, i. 592, 602
- — chloride, i. 602
- — crystallohydrates of, i. 601
- — double salts of, i. 597
- — nitride, i. 595
- — silicide, ii. 102
- — sulphate, i. 600
- Manganese, ii. 303
- — nitrides, ii. 310
- — oxides, ii. 306, 307, 308, 313
- — peroxide, i. 159; ii. 305
- — sulphate, ii. 307
- Mass, influence of, i. 32, 436
- Matches, ii. 154
- Matter, primary, ii. 440
- — transmutability of, i. 14
- Mercury, ii. 48
- — ammonia compounds, ii. 57
- — basic salts of, ii. 54
- — chlorides, ii. 52, 53, 54
- — compounds, heat of formation, ii. 50
- — cyanide, ii. 55
- — fulminating, ii. 56
- — iodide, ii. 55
- — nitrates, ii. 51
- — nitrides, ii. 56
- — oxides, ii. 53
- — sulphate, ii. 57
- — sulphides, ii. 221
- Metalepsis, i. 28, 471
- Metalloids, i. 23
- Metals, i. 23
- — of alkaline earths, i. 64, 590, 591
- — of alkalis, i. 543
- — displacement of, ii. 427
- Methane, i. 360
- Moisture, determination of, in gases, i. 40
- — influence upon reaction, i. 403
- Molecular volumes, ii. 37
- — weight and boiling point, i. 331
- — — — coefficient of refraction, i. 336
- — — — latent heat, i. 329
- — — — specific gravity of solutions, i. 335
- — — — surface tension, i. 334[515]
- Molecules, i. 319, 322
- Molybdates, ii. 292
- Molybdenum, ii. 290
- — anhydride, ii. 291
- — fluo-compounds, ii. 298
- — sulphides, ii. 297
- Monophosphamide, ii. 178
- Monosodium orthophosphate, ii. 167
- Morphotropy, ii. 10
- Naphtha, i. 373, 377
- Nascent state, i. 33, 145, 146
- Neodymium, ii. 97
- Nickel, ii. 353
- — alloys, ii. 367
- — and carbonic oxide, ii. 367
- — fluoride, ii. 358
- — hydroxide, ii. 358
- — oxide, ii. 365
- — sulphate, i. 97; ii. 359
- — tetra-carboxyl, ii. 367
- Niobium, i. 199; ii. 194, 198
- Nitrates, i. 273
- Nitres, i. 268, 555
- Nitric anhydride, i. 280
- — oxide, i. 286
- Nitrides, i. 227, 258, 620
- Nitriles, i. 406
- Nitrites, i. 284
- Nitro-cellulose, i. 275
- Nitro-compounds, i. 274
- Nitrogen, i. 223, 225, 475
- — chloride, i. 476
- — iodide, i. 507
- — oxides of, i. 267, 280, 284, 294, 295
- — sulphide, ii. 270
- Nitro-prussides, ii. 351
- Nitroso-compounds, i. 288
- Nitrosulphates, ii. 229
- Nitrosyl chloride, ii. 176
- Norwegium, ii. 59
- Occlusion, i. 143
- Olefiant gas, i. 370
- Organo-metallic compounds, i. 356
- Osmium, ii. 372, 382, 384
- Osmotic pressure, i. 64
- Osmuridium, ii. 383
- Oxamide, i. 406
- Oxidation, i. 16
- Oxides, i. 183; ii. 36
- Oxycobaltamine salts, ii. 359
- Oxygen, i. 152, 157, 158, 163
- — compounds, heat of formation of, i. 120, 466
- Ozone, i. 198, 229
- Palladium, ii. 369
- — hydride, i. 143; ii. 380
- Palladous chloride, ii. 379
- — iodide, ii. 379
- Paracyanogen, i. 414
- Paramorphism, ii. 9
- Parasulphatammon, ii. 269
- Peat, i. 344
- Peligot's salt, ii. 281
- Percentage composition, i. 326
- Perchloric anhydride, ii. 282
- Periodates, i. 510
- Permanganic anhydride, ii. 313
- Permolybdates, ii. 297
- Peroxide, chloric, i. 484
- Peroxides, i. 159; ii. 15, 23
- Perstannic oxide, ii. 133
- Persulphates, ii. 253
- Petroleum, i. 373
- Phenol, solubility of, i. 75
- Phlogiston, i. 17
- Phosgene gas, ii. 175
- Phospham, ii. 178
- Phosphides, ii. 157
- Phosphine, ii. 158, 160
- Phosphonium iodide, ii. 159
- Phosphoric anhydride, ii. 161
- Phosphorous anhydride, ii. 160
- Phosphorus, ii. 149
- — ammonium compounds, ii. 178
- — chlorides, ii. 174
- — fluorides, ii. 173
- — iodides, i. 505, 506; ii. 172
- — oxychlorides, ii. 175
- — sulphides, ii. 213
- — sulphochloride, ii. 213
- — thermo-chemical data for, ii. 153
- Phosphuretted hydrogen, ii. 158, 160
- Photography, ii. 431
- Photo-salts, ii. 432
- Plants, chemical reactions in, i. 547
- — and nitrogen, i. 230
- Platinic chloride, ii. 377
- — hydroxide, ii. 379
- Platino-ammonium compounds, ii. 391
- — chlorides, i. 467; ii. 378
- — cyanides, ii. 386
- — nitrites, ii. 390
- — sulphites, ii. 390
- Platinous chloride, ii. 379
- Platinum, ii. 376
- — alloys, ii. 373
- — black, ii. 376
- — metals, ii. 369, 375
- — oxide, ii. 378
- Poly-haloid salts, i. 545
- Polymerism, i. 207, 367
- Polysulphides, ii. 217
- Potassium, i. 544, 558
- — aurate, ii. 449
- — bromide, i. 550[516]
- Potassium carbonate, i. 549
- — chlorate, i. 161, 482
- — chloride, i. 72, 543
- — chromate, ii. 280
- — cyanide, i. 412, 551
- — dichromate, ii. 278
- — ferricyanide, ii. 346
- — ferrocyanide, i. 346, 412
- — hydrosulphide, ii. 219
- — hydroxide, i. 548
- — iodide, i. 550
- — manganate, ii. 310
- — nitrate, i. 553
- — oxides, i. 559
- — permanganate, ii. 311
- — stannate, ii. 133
- — sulphate, i. 72, 549
- — sulphide, ii. 219
- — telluride, ii. 274
- Praseocobaltic salts, ii. 361
- Praseodidymium, ii. 97
- Proteid substances, i. 224
- Prout's hypothesis, ii. 439
- Prussian blue, i. 419; ii. 349
- Purpureocobaltic salts, ii. 361
- Purpureotetramine salts, ii. 361
- Pyrocollodion, i. 275
- Pyronaphtha, i. 375
- Pyrosulphuryl chloride, i. 321; ii. 235
- Reactions, chemical, i. 3
- — — conditions for, i. 34
- — — contact, i. 39
- — — endothermal, i. 30
- — — exothermal, i. 30
- — — limit of, i. 437
- — — rate of, ii. 152
- Recalescence, ii. 333
- Reduction, i. 16
- Refraction equivalent, i. 336
- Regenerative furnaces, i. 398
- Reiset's salts, ii. 394
- Respiration, i. 152, 154, 387
- Rhodium, ii. 381
- Rock salt, i. 421
- Roseocobaltic salts, ii. 360
- Rosetetramine salts, ii. 361
- Rubidium, i. 576
- Ruthenium, ii. 372, 382, 384
- Sal-ammoniac, i. 248, 318, 457
- — solubility of, i. 458
- — vapour density of, i. 317
- Salts, i. 187, 419
- — acid, i. 193, 533
- — basic, i. 193, 533; ii. 54
- — double, i. 598
- — electrolysis of, i. 191
- — heat of formation, i. 189
- — melting points of, i. 135
- — pyro, i. 193
- — theory of, i. 193
- Saponification, i. 530
- Scandium, ii. 94
- Selenium, ii. 273
- — chlorides, ii. 275
- Selenious anhydride, ii. 271
- Silica, i. 100 ii. 108
- — soluble, ii. 113
- Silicates, i. 544; ii. 116
- Silicon, ii. 99
- — chloride, ii. 103, 104
- — chloroform, ii. 103
- — bromide, ii. 104
- — fluoride, ii. 105
- — hydride, ii. 102, 103
- — iodide, ii. 105
- — iodoform, ii. 105
- Silver, ii. 418
- — allotropic varieties of, ii. 421
- — bromide, ii. 429
- — chlorate, ii. 437
- — chloride, ii. 429
- — cyanide, ii. 433
- — fluoride, ii. 430
- — fulminating, ii. 426
- — hyponitrite, i. 294
- — iodide, ii. 429
- — nitrate, ii. 426
- — nitrite, i. 284
- — orthophosphate, ii. 164
- — oxides, ii. 424
- — peroxide, ii. 422
- — plating, ii. 434
- — soluble, ii. 420
- — subchloride, ii. 432
- Slags, ii. 323
- Smalt, ii. 354
- Soaps, i. 531
- Soda ash, i. 519
- — caustic, i. 527
- — manufacture of, i. 459
- — waste, i. 522
- Soda lime, i. 237
- Sodamide, i. 539
- Sodium, i. 513, 533
- — alloys, i. 559
- — amalgams, i. 537
- — bicarbonate, i. 526
- — carbonate, i. 519, 525
- — — crystallohydrates of, i. 108
- — — manufacture of, i. 523
- — — solutions of, i. 525
- — chloride, i. 419
- — — double salts of, i. 430
- — — solutions of, i. 88, 99, 429[517]
- Sodium hydride, i. 537
- — hydroxide, i. 528, 529
- — — solutions of, i. 529
- — nitrate, i. 269
- — — solutions of, i. 72
- — organo compounds of, i. 540
- — oxides, i. 540, 541
- — phosphates, ii. 166
- — platinate, ii. 378
- — pyrosulphate, i. 518
- — sesquicarbonate, i. 526
- — stannate, ii. 133
- — subchloride, i. 540
- — sulphate, i. 513
- — — acid salt, i. 518
- — — crystallohydrates of, i. 515
- — — solutions of, i. 73, 515, 516
- — sulphite, ii. 226
- — thiosulphate, ii. 230
- — — solutions of, i. 74
- — tungstate, ii. 294
- Soils, i. 344; ii. 73
- Solubility, coefficient of, i. 67, 71
- Solutions, i. 330
- — aqueous, i. 59
- — boiling points of, i. 94, 100
- — crystallisation of, i. 427
- — colour of, i. 95
- — diffusion of, i. 61, 429
- — of double salts, i. 599
- — formation of ice from, i. 91, 428
- — heat of formation of, i. 74, 75, 76
- — of gases, i. 68
- — isotonic, i. 64
- — saturated, i. 65
- — specific gravity of, i. 429, 584
- — supersaturated, i. 96
- — theory of, i. 64, 89, 92, 97, 106, 215, 323, 608; ii. 3, 164
- — vapour tension of, i. 90, 92
- — volumes of, i. 87
- — Specific heat, i. 585, 586, 588
- Spectra absorption, i. 566
- Spectrum analysis, i. 560, 561
- Stannic chloride, ii. 132
- — fluoride, ii. 132
- — oxide, ii. 130
- — sulphide, ii. 132
- Stannous chloride, ii. 130
- — oxide, ii. 129
- — salts, ii. 129
- Steam, vapour tension of, i. 54
- Steel, ii. 327, 328, 330
- Strontium, i. 615
- — chloride, i. 615
- — hydroxide, i. 615
- — nitrate, i. 615
- — oxide, i. 617
- Substitution chemical, i. 5
- Sulphamide, ii. 270
- Sulphatammon, ii. 269
- Sulphates, ii. 248
- Sulphides, i. 98; ii. 213
- Sulphonitrites, ii. 229
- Sulphoxyl, ii. 250
- Sulphur, ii. 200
- — chlorides of, ii. 264
- Sulphuretted hydrogen, ii. 208
- Sulphuric anhydride, ii. 232
- — peroxide, ii. 251
- Sulphurous anhydride, ii. 224
- Sulphuryl chloride, ii. 268
- Superphosphates, ii. 168
- Tantalum, ii. 194, 198
- Tellurium, ii. 274
- — bromide, ii. 275
- — chlorides, ii. 275
- Tellurous anhydride, ii. 271
- Temperature, critical, i. 131
- Test papers, i. 185
- Thallium, ii. 88, 91
- Thallic oxide, ii. 93
- Thallous hydroxide, ii. 92
- — oxide, ii. 92
- Thiocarbonates, ii. 262
- Thionyl chloride, ii. 267
- Thiophosgene, ii. 262
- Thiophosphoryl fluoride, ii. 263
- Theory, atomic, i. 216
- — unitary, i. 195
- — vortex, i. 217
- Thermochemistry, i. 173
- Thorium, ii. 148
- Tin, ii. 125
- — alloys, ii. 127
- Titanium, ii. 144
- — chloride, ii. 145
- — nitride, ii. 146
- — nitrocyanide, ii. 146
- — oxides, ii. 145
- Tripoli, ii. 110
- Trisodium orthophosphate, ii. 166
- Tungstates, ii. 292
- Tungsten, ii. 290
- — anhydride, ii. 291
- — nitride, ii. 297
- — sulphide, ii. 297
- Turnbull's blue, ii. 350
- Types of combination, ii. 10
- Ultramarine, ii. 84
- Uranium, ii. 30, 297
- — atomic weight of, ii. 26
- — dioxide, ii. 301
- — oxides, ii. 298[518]
- Uranium tetrachloride, ii. 301
- Urano-alkali compounds, ii. 298
- Uranyl, ii. 301
- — ammonium carbonate, ii. 300
- — nitrate, ii. 300
- — phosphate, ii. 300
- Urea, i. 409
- Valency of elements, i. 404, 418, 581
- Van der Waal's formula, i. 82, 140
- Vanadic anhydride, ii. 196
- Vanadium, ii. 194
- — oxychloride, ii. 195
- Vapour density, determination of, i. 301
- Ventilation, i. 244
- Viscosity, i. 355
- Volumes, molecular, ii. 4
- — gases, i. 300
- Water, i. 40
- — composition of, i. 114, 118, 148, 169, 305, 333
- — compressibility of, i. 53
- — of constitution, i. 109
- — of crystallisation, i. 95, 510
- — dissociation of, i. 118
- — expansion of, i. 53
- Water gas, i. 129, 400, 401
- — hard, i. 47
- — hygroscopic, i. 56
- — mineral, i. 45
- — rain, i. 43
- — river, i. 43
- — sea, i. 46
- — specific heat of, i. 52
- — — gravity of, i. 50
- — spring, i. 44
- Wave lengths, i. 564
- Wood, i. 339
- Ytterbium, ii. 93
- Yttrium, ii. 93
- Zinc, ii. 39
- — ammonia-chlorides, ii. 41
- — chloride, ii. 40, 41
- — compounds, heat of formation of, ii. 51
- — oxide, ii. 39, 40
- — sulphate, ii. 39
- Zirconium, ii. 146
- — chloride, ii. 147
- — hydroxide, ii. 147
- — oxide, ii. 147
PRINTED BY
SPOTTISWOODE AND CO., NEW-STREET SQUARE
LONDON
A
CLASSIFIED CATALOGUE
OF
SCIENTIFIC WORKS
PUBLISHED BY
MESSRS. LONGMANS, GREEN, & CO.
LONDON: 39 PATERNOSTER ROW, E.C.
NEW YORK: 91 & 93 FIFTH AVENUE.
BOMBAY: 32 HORNBY ROAD.
CONTENTS.
| |
PAGE |
| Advanced Science Manuals |
30 |
| Agriculture and Gardening |
26 |
| Astronomy |
14 |
| Biology |
24 |
| Botany |
25 |
| Building Construction |
10 |
| Chemistry |
2 |
| Dynamics |
6 |
| Electricity |
11 |
| Elementary Science Manuals |
30 |
| Engineering |
12 |
| Evolution |
18 |
| Geology |
18 |
| Health and Hygiene |
17 |
| Heat |
8 |
| Hydrostatics |
6 |
| Light |
8 |
| London Science Class-Books |
32 |
| Longmans' Civil Engineering Series |
13 |
| Machine Drawing and Design |
13 |
| Magnetism |
11 |
| Manufactures |
17 |
| Mechanics |
6 |
| Medicine and Surgery |
19 |
| Metallurgy |
14 |
| Mineralogy |
14 |
| Natural History |
18 |
| Navigation |
14 |
| Optics |
8 |
| Photography |
8 |
| Physics |
5 |
| Physiography |
17 |
| Physiology |
24 |
| Proctor's (R. A.) Works |
15 |
| Sound |
8 |
| Statics |
6 |
| Steam, Oil, and Gas Engines |
9 |
| Strength of Materials |
12 |
| Technology |
17 |
| Telegraphy |
12 |
| Telephone |
12 |
| Text-Books of Science |
28 |
| Thermodynamics |
8 |
| Tyndall's (John) Works |
27 |
| Workshop Appliances |
14 |
[2]
CHEMISTRY.
CORNISH.—PRACTICAL PROOFS OF CHEMICAL LAWS:
A Course of Experiments upon the Combining Proportions of the Chemical
Elements. By Vaughan Cornish, M.Sc., Associate of the Owens College,
Manchester. Crown 8vo., 2s.
CROOKES.—SELECT METHODS IN CHEMICAL
ANALYSIS, chiefly Inorganic. By William Crookes, F.R.S., etc. Third
Edition, Rewritten and Enlarged. With 67 Woodcuts. 8vo., 21s. net.
CROSS AND BEVAN.—CELLULOSE: an Outline of the
Chemistry of the Structural Elements of Plants. With Reference to their
Natural History and Industrial Uses. By Cross and Bevan (C. F. Cross, E.
J. Bevan, and C. Beadle). With 14 Plates. Crown 8vo., 12s. net.
FURNEAUX.—ELEMENTARY CHEMISTRY, Inorganic and
Organic. By W. Furneaux, F.R.C.S., Lecturer on Chemistry, London
School Board. With 65 Illustrations and 155 Experiments. Crown 8vo., 2s. 6d.
HJELT.—PRINCIPLES OF GENERAL ORGANIC CHEMISTRY.
By Professor E. Hjelt, of Helsingfors. Translated from the
German by J. Bishop Tingle, Ph.D., Assistant in the Laboratory of the
Heriot Watt College, Edinburgh. Crown 8vo., 6s. 6d.
JAGO.—Works by W. JAGO, F.C.S., F.I.C.
INORGANIC CHEMISTRY, THEORETICAL AND
PRACTICAL. With an Introduction to the Principles of Chemical Analysis,
Inorganic and Organic. With 63 Woodcuts and numerous Questions and
Exercises. Fcp. 8vo., 2s. 6d.
AN INTRODUCTION TO PRACTICAL INORGANIC
CHEMISTRY. Crown 8vo., 1s. 6d.
INORGANIC CHEMISTRY, THEORETICAL AND
PRACTICAL. A Manual for Students in Advanced Classes of the Science
and Art Department. With Plate of Spectra and 78 Woodcuts. Crown
8vo., 4s. 6d.
KOLBE.—A SHORT TEXTBOOK OF INORGANIC
CHEMISTRY. By Dr. Hermann Kolbe. Translated and Edited by T. S.
Humpidge, Ph.D. With 66 Illustrations. Crown 8vo., 8s. 6d.
MENDELÉEFF.—THE PRINCIPLES OF CHEMISTRY.
By D. Mendeléeff, Professor of Chemistry in the University of St. Petersburg.
Translated by George Kamensky, A.R.S.M., of the Imperial Mint,
St. Petersburg, and Edited by A. J. Greenaway, F.I.C., Sub-Editor of the
Journal of the Chemical Society. With 97 Illustrations. 2 vols. 8vo., 36s.
MEYER.—OUTLINES OF THEORETICAL CHEMISTRY.
By Lothar Meyer, Professor of Chemistry in the University of Tübingen.
Translated by Professors P. Phillips Bedson, D.Sc., and W. Carleton
Williams, B.Sc. 8vo., 9s.
MILLER.—INTRODUCTION TO THE STUDY OF INORGANIC
CHEMISTRY. By W. Allen Miller, M.D., LL.D. With
71 Woodcuts. Fcp. 8vo., 3s. 6d.
[3]
NEWTH.—Works by G. S. NEWTH, F.I.C., F.C.S., Demonstrator
in the Royal College of Science, London; Assistant
Examiner in Chemistry, Science and Art Department.
CHEMICAL LECTURE EXPERIMENTS, NON-METALLIC
ELEMENTS. With 230 Diagrams. Crown 8vo., 10s. 6d.
A TEXT-BOOK OF INORGANIC CHEMISTRY. With 146
Illustrations. Crown 8vo., 6s. 6d.
ELEMENTARY PRACTICAL CHEMISTRY: a Laboratory
Manual for Use in Organised Science Schools. With 108 Illustrations and
254 Experiments. Crown 8vo., 2s. 6d.
OSTWALD.—SOLUTIONS. By W. Ostwald, Professor of
Chemistry in the University of Leipzig. Being the Fourth Book, with some
additions, of the Second Edition of Ostwald's ‘Lehrbuch der allgemeinen
Chemie’. Translated by M. M. Pattison Muir, Fellow of Gonville and
Caius College, Cambridge. 8vo., 10s. 6d.
PAYEN.—INDUSTRIAL CHEMISTRY. A Manual for use in
Technical Colleges and Schools, based upon a Translation of Stohmann and
Engler's German Edition of Payen's Précis de Chimie Industrielle. Edited
by B. H. Paul, Ph.D. With 698 Woodcuts. 8vo., 42s.
REYNOLDS.—EXPERIMENTAL CHEMISTRY FOR
JUNIOR STUDENTS. By J. Emerson Reynolds, M.D., F.R.S., Professor
of Chemistry, University of Dublin; Examiner in Chemistry, University
of London. Fcp. 8vo., with numerous Woodcuts.
Part I. Introductory. Fcp. 8vo., 1s. 6d.
Part II. Non-Metals, with an Appendix on Systematic Testing
for Acids. Fcp. 8vo., 2s. 6d.
Part III. Metals, and Allied Bodies. Fcp. 8vo., 3s. 6d.
Part IV. Carbon Compounds. Fcp. 8vo., 4s.
SHENSTONE.—Works by W. A. SHENSTONE, Lecturer on
Chemistry in Clifton College.
THE METHODS OF GLASS-BLOWING. For the use of
Physical and Chemical Students. With 42 Illustrations. Crown 8vo., 1s. 6d.
A PRACTICAL INTRODUCTION TO CHEMISTRY.
Intended to give a Practical acquaintance with the Elementary Facts and
Principles of Chemistry. With 25 Illustrations. Crown 8vo., 2s.
[4]
THORPE.—A DICTIONARY OF APPLIED CHEMISTRY.
By T. E. Thorpe, B.Sc. (Vict.), Ph.D., F.R.S., Treas. C.S., Professor of
Chemistry in the Royal College of Science, South Kensington. Assisted by
Eminent Contributors. 3 vols. 8vo. Vols. I. and II., 42s. each. Vol. III., 63s.
THORPE.—QUANTITATIVE CHEMICAL ANALYSIS. By
T. E. Thorpe, Ph.D., F.R.S. With 88 Woodcuts. Fcp. 8vo., 4s. 6d.
THORPE AND MUIR.—QUALITATIVE CHEMICAL ANALYSIS
AND LABORATORY PRACTICE. By T. E. Thorpe, Ph.D.,
D.Sc., F.R.S., and M. M. Pattison Muir, M.A. With Plate of Spectra and
57 Woodcuts. Fcp. 8vo., 3s. 6d.
TILDEN.—Works by WILLIAM A. TILDEN, D.Sc. London,
F.R.S., Professor of Chemistry in the Royal College of Science,
London.
INTRODUCTION TO THE STUDY OF CHEMICAL
PHILOSOPHY. The Principles of Theoretical and Systematic Chemistry.
With 5 Woodcuts. With or without the ANSWERS of Problems. Fcp.
8vo., 4s. 6d.
PRACTICAL CHEMISTRY. The principles of Qualitative
Analysis. Fcp. 8vo., 1s. 6d.
HINTS ON THE TEACHING OF ELEMENTARY
CHEMISTRY IN SCHOOLS AND SCIENCE CLASSES. With 7
Illustrations. Crown 8vo., 2s.
WATTS' DICTIONARY OF CHEMISTRY. Revised and
entirely Re-written by H. Forster Morley, M.A., D.Sc., Fellow of, and
lately Assistant-Professor of Chemistry in University College, London; and M.
M. Pattison Muir, M.A., F.R.S.E., Fellow, and Prælector in Chemistry, of
Gonville and Caius College, Cambridge. Assisted by Eminent Contributors.
4 vols. 8vo. Vols. I. and II., 42s. each. Vol. III., 50s. Vol. IV., 63s.
WHITELEY.—Works by R. LLOYD WHITELEY, F.I.C.,
Principal of the Municipal Science School, West Bromwich.
CHEMICAL CALCULATIONS. With Explanatory Notes,
Problems and Answers, specially adapted for use in Colleges and Science
Schools. With a Preface by Professor F. Clowes, D.Sc. (Lond.), F.I.C.
Crown 8vo., 2s.
ORGANIC CHEMISTRY: the Fatty Compounds. With 45
Illustrations. Crown 8vo., 3s. 6d.
[5]
PHYSICS, ETC.
EARL.—THE ELEMENTS OF LABORATORY WORK:
a Course of Natural Science. By A. G. Earl, M.A., F.C.S., late Scholar of
Christ's College, Cambridge. With 57 Diagrams and numerous Exercises and
Questions. Crown 8vo., 4s. 6d.
GANOT.—Works by PROFESSOR GANOT. Translated and
Edited by E. ATKINSON, Ph.D., F.C.S.
ELEMENTARY TREATISE ON PHYSICS, Experimental
and Applied. With 9 Coloured Plates and Maps, and 1028 Woodcuts, and
Appendix of Problems and Examples with Answers. Crown 8vo., 15s.
NATURAL PHILOSOPHY FOR GENERAL READERS
AND YOUNG PERSONS. With 7 Plates, 624 Woodcuts, and an Appendix
of Questions. Crown 8vo., 7s. 6d.
GLAZEBROOK AND SHAW.—PRACTICAL PHYSICS. By
R. T. Glazebrook, M.A., F.R.S., and W. N. Shaw, M.A. With 134
Woodcuts. Fcp. 8vo., 7s. 6d.
GUTHRIE.—MOLECULAR PHYSICS AND SOUND. By
F. Guthrie, Ph.D. With 91 Diagrams. Fcp. 8vo., 1s. 6d.
PHYSICAL AND ELECTRICAL ENGINEERING LABORATORY MANUALS.—Vol. 1.
HENDERSON.—ELEMENTARY PHYSICS. By John
Henderson, B.Sc. (Edin.), A.I.E.E., Lecturer in Physics, Manchester Municipal
Technical School. Crown 8vo., 2s. 6d.
HELMHOLTZ.—POPULAR LECTURES ON SCIENTIFIC
SUBJECTS. By Hermann von Helmholtz. Translated by E. Atkinson,
Ph.D., F.C.S., formerly Professor of Experimental Science, Staff College. With
68 Illustrations. 2 vols., crown 8vo., 3s. 6d. each.
Contents.—Vol. I.—The Relation of Natural Science to Science in General—Goethe's
Scientific Researches—The Physiological Causes of Harmony in Music—Ice and Glaciers—The
Interaction of the Natural Forces—The Recent Progress of the Theory of Vision—The Conservation
of Force—The Aim and Progress of Physical Science.
Contents.—Vol. II.—Gustav Magnus. In Memoriam—The Origin and Significance of
Geometrical Axioms—The Relation of Optics to Painting—The Origin of the Planetary System—Thought
in Medicine—Academic Freedom in German Universities—Hermann Von Helmholtz—An
Autobiographical Sketch.
WATSON.—ELEMENTARY PRACTICAL PHYSICS: a
Laboratory Manual for Use in Organised Science Schools. By W. Watson
B.Sc. Demonstrator in Physics in the Royal College of Science, London;
Assistant Examiner in Physics, Science and Art Department. With 119 Illustrations
and 193 Exercises. Crown 8vo., 2s. 6d.
[6]
WORTHINGTON.—A FIRST COURSE OF PHYSICAL
LABORATORY PRACTICE. Containing 264 Experiments. By A. M.
Worthington, M.A., F.R.S. With Illustrations. Crown 8vo., 4s. 6d.
WRIGHT.—ELEMENTARY PHYSICS. By Mark R.
Wright, Professor of Normal Education, Durham College of Science. With
242 Illustrations. Crown 8vo., 2s. 6d.
MECHANICS, DYNAMICS, STATICS, HYDROSTATICS, ETC.
BALL.—A CLASS-BOOK OF MECHANICS. By Sir R. S.
Ball, LL.D. 89 Diagrams. Fcp. 8vo., 1s. 6d.
GELDARD.—STATICS AND DYNAMICS. By C. Geldard,
M.A., formerly Scholar of Trinity College, Cambridge. Crown 8vo., 5s.
GOODEVE.—Works by T. M. GOODEVE, M.A., formerly
Professor of Mechanics at the Normal School of Science, and
the Royal School of Mines.
THE ELEMENTS OF MECHANISM. With 342 Woodcuts.
Crown 8vo., 6s.
PRINCIPLES OF MECHANICS. With 253 Woodcuts and
numerous Examples. Crown 8vo., 6s.
A MANUAL OF MECHANICS: an Elementary Text-Book
for Students of Applied Mechanics. With 138 Illustrations and Diagrams,
and 188 Examples taken from the Science Department Examination Papers,
with Answers. Fcp. 8vo., 2s. 6d.
GRIEVE.—LESSONS IN ELEMENTARY MECHANICS.
By W. H. Grieve, P.S.A., late Engineer, R.N., Science Demonstrator for the
London School Board, etc.
Stage 1. With 165 Illustrations and a large number of Examples. Fcp. 8vo.,
1s. 6d.
Stage 2. With 122 Illustrations. Fcp. 8vo., 1s. 6d.
Stage 3. With 103 Illustrations. Fcp. 8vo., 1s. 6d.
[7]
MAGNUS.—Works by SIR PHILIP MAGNUS, B.Sc., B.A.
LESSONS IN ELEMENTARY MECHANICS. Introductory
to the study of Physical Science. Designed for the Use of Schools, and of
Candidates for the London Matriculation and other Examinations. With
numerous Exercises, Examples, Examination Questions, and Solutions, etc.,
from 1870–1895. With Answers, and 131 Woodcuts. Fcp. 8vo., 3s. 6d.
Key for the use of Teachers only, price 5s. 3½d.
HYDROSTATICS AND PNEUMATICS. Fcp. 8vo., 1s. 6d.;
or, with Answers, 2s. The Worked Solutions of the Problems, 2s.
ROBINSON.—ELEMENTS OF DYNAMICS (Kinetics and
Statics). With numerous Exercises. A Text-book for Junior Students. By
the Rev. J. L. Robinson, B.A. Crown 8vo., 6s.
SMITH.—Works by J. HAMBLIN SMITH, M.A.
ELEMENTARY STATICS. Crown 8vo., 3s.
ELEMENTARY HYDROSTATICS. Crown 8vo., 3s.
KEY TO STATICS AND HYDROSTATICS. Crown 8vo., 6s.
TATE.—EXERCISES ON MECHANICS AND NATURAL
PHILOSOPHY. By Thomas Tate, F.R.A.S. Fcp. 8vo., 2s. Key, 3s. 6d.
TAYLOR.—Works by J. E. TAYLOR, M.A., B.Sc. (Lond.),
Head Master of the Central Higher Grade and Science School,
Sheffield.
THEORETICAL MECHANICS, including Hydrostatics and
Pneumatics. With 175 Diagrams and Illustrations, and 522 Examination
Questions and Answers. Crown 8vo., 2s. 6d.
THEORETICAL MECHANICS.—SOLIDS. With 163 Illustrations,
120 Worked Examples and over 500 Examples from Examination
Papers, etc. Crown 8vo., 2s. 6d.
THEORETICAL MECHANICS.—FLUIDS. With 122 Illustrations,
numerous Worked Examples, and about 500 Examples from Examination
Papers, etc. Crown 8vo., 2s. 6d.
THORNTON.—THEORETICAL MECHANICS—SOLIDS.
Including Kinematics, Statics, and Kinetics. By Arthur Thornton, M.A.,
F.R.A.S. With 200 Illustrations, 130 Worked Examples, and over 900
Examples from Examination Papers, etc. Crown 8vo., 4s. 6d.
[8]
TWISDEN.—Works by the Rev. JOHN F. TWISDEN, M.A.
PRACTICAL MECHANICS; an Elementary Introduction to
their Study. With 855 Exercises, and 184 Figures and Diagrams. Crown
8vo., 10s. 6d.
THEORETICAL MECHANICS. With 172 Examples,
numerous Exercises, and 154 Diagrams. Crown 8vo., 8s. 6d.
WILLIAMSON.—INTRODUCTION TO THE MATHEMATICAL
THEORY OF THE STRESS AND STRAIN OF ELASTIC
SOLIDS. By Benjamin Williamson, D.Sc., F.R.S. Crown 8vo., 5s.
WILLIAMSON AND TARLETON.—AN ELEMENTARY
TREATISE ON DYNAMICS. Containing Applications to Thermodynamics,
with numerous Examples. By Benjamin Williamson, D.Sc., F.R.S., and
Francis A. Tarleton, LL.D. Crown 8vo., 10s. 6d.
WORTHINGTON.—DYNAMICS OF ROTATION: an Elementary
introduction to Rigid Dynamics. By A. M. Worthington, M.A.,
F.R.S. Crown 8vo., 3s. 6d.
OPTICS AND PHOTOGRAPHY.
ABNEY.—A TREATISE ON PHOTOGRAPHY. By Captain
W. de Wiveleslie Abney, F.R.S., Director for Science in the Science and
Art Department. With 115 Woodcuts. Fcp. 8vo., 3s. 6d.
GLAZEBROOK.—PHYSICAL OPTICS. By R. T. Glazebrook,
M.A., F.R.S., Fellow and Lecturer of Trinity College, Demonstrator
of Physics at the Cavendish Laboratory, Cambridge. With 183 Woodcuts of
Apparatus, etc. Fcp. 8vo., 6s.
WRIGHT.—OPTICAL PROJECTION: a Treatise on the Use
of the Lantern in Exhibition and Scientific Demonstration. By Lewis Wright,
Author of ‘Light: a Course of Experimental Optics’. With 232 Illustrations.
Crown 8vo., 6s.
SOUND, LIGHT, HEAT, AND THERMODYNAMICS.
ALEXANDER.—TREATISE ON THERMODYNAMICS.
By Peter Alexander, M.A. Crown 8vo., 5s.
CUMMING.—HEAT. For the Use of Schools and Students.
By Linnæus Cumming, M.A. With numerous Illustrations. Crown 8vo.,
4s. 6d.
DAY.—NUMERICAL EXAMPLES IN HEAT. By R. E.
Day, M.A. Fcp. 8vo., 3s. 6d.
[9]
EMTAGE.—LIGHT. By W. T. A. Emtage, M.A. With
232 Illustrations. Crown 8vo., 6s.
HELMHOLTZ.—ON THE SENSATIONS OF TONE AS A
PHYSIOLOGICAL BASIS FOR THE THEORY OF MUSIC. By Hermann
Von Helmholtz. Royal 8vo., 28s.
MADAN.—AN ELEMENTARY TEXT-BOOK ON HEAT.
For the Use of Schools. By H. G. Madan, M.A., F.C.S., Fellow of Queen's
College, Oxford; late Assistant Master at Eton College. Crown 8vo., 9s.
MAXWELL.—THEORY OF HEAT. By J. Clerk Maxwell,
M.A., F.R.SS., L. and E. With Corrections and Additions by Lord Rayleigh.
With 38 Illustrations. Fcp. 8vo., 4s. 6d.
SMITH.—THE STUDY OF HEAT. By J. Hamblin Smith,
M.A., of Gonville and Caius College, Cambridge. Crown 8vo., 3s.
TYNDALL.—Works by JOHN TYNDALL, D.C.L., F.R.S.
See p. 27.
WORMELL.—A CLASS-BOOK OF THERMODYNAMICS.
By Richard Wormell, B.Sc., M.A. Fcp. 8vo., 1s. 6d.
WRIGHT.—Works by MARK R. WRIGHT, Hon. Inter. B.Sc.,
London.
SOUND, LIGHT, AND HEAT. With 160 Diagrams and
Illustrations. Crown 8vo., 2s. 6d.
ADVANCED HEAT. With 136 Diagrams and numerous
Examples and Examination Papers. Crown 8vo., 4s. 6d.
STEAM, OIL, AND GAS ENGINES.
BALE.—A HANDBOOK FOR STEAM USERS; being Rules
for Engine Drivers and Boiler Attendants, with Notes on Steam Engine and
Boiler Management and Steam Boiler Explosions. By M. Powis Bale,
M.I.M.E., A,M.I.C.E. Fcp. 8vo., 2s. 6d.
BOLTON.—MOTIVE POWERS AND THEIR PRACTICAL
SELECTION. By Reginald Bolton, Associate Member of the Institution
of Civil Engineers, etc. Crown 8vo., 6s. 6d. net.
CLERK.—THE GAS AND OIL ENGINE. By Dugald
Clerk, Associate Member of the Institution of Civil Engineers, Fellow of the
Chemical Society, Member of the Royal Institution, Fellow of the Institute of
Patent Agents. With 228 Illustrations and Diagrams. 8vo., 15s.
HOLMES.—THE STEAM ENGINE. By George C. V.
Holmes, Whitworth Scholar, Secretary of the Institution of Naval Architects.
With 212 Woodcuts. Fcp. 8vo., 6s.
[10]
NORRIS.—A PRACTICAL TREATISE ON THE ‘OTTO’
CYCLE GAS ENGINE. By William Norris, M.I.Mech.E. With 207
Illustrations. 8vo., 10s. 6d.
RIPPER.—STEAM. By William Ripper, Member of the
Institution of Mechanical Engineers; Professor of Mechanical Engineering in
the Sheffield Technical School. With 142 Illustrations. Crown 8vo., 2s. 6d.
SENNETT.—THE MARINE STEAM ENGINE. A Treatise
for the Use of Engineering Students and Officers of the Royal Navy. By
Richard Sennett, R.N., late Engineer-in-Chief of the Royal Navy. With
261 Illustrations. 8vo., 21s.
STROMEYER.—MARINE BOILER MANAGEMENT AND
CONSTRUCTION. Being a Treatise on Boiler Troubles and Repairs,
Corrosion, Fuels, and Heat, on the proporties of Iron and Steel, on Boiler
Mechanics, Workshop Practices, and Boiler Design. By C. E. Stromeyer,
Graduate of the Royal Technical College at Aix-la-Chapelle, Member of the
Institute of Naval Architects, etc. 8vo., 18s. net.
BUILDING CONSTRUCTION.
ADVANCED BUILDING CONSTRUCTION. By the Author
of ‘Rivington's’ Notes on Building Construction'. With 385 Illustrations.
Crown 8vo., 4s. 6d.
BURRELL.—BUILDING CONSTRUCTION. By Edward J.
Burrell, Second Master of the People's Palace Technical School, London.
With 303 Working Drawings. Crown 8vo., 2s. 6d.
SEDDON.—BUILDER'S WORK AND THE BUILDING
TRADES. By Col. H. C. Seddon, R.E., Superintending Engineer, H.M.'s
Dockyard, Portsmouth; Examiner in Building Construction, Science and Art
Department, South Kensington; with numerous Illustrations. Medium 8vo.,
16s.
RIVINGTON'S COURSE OF BUILDING CONSTRUCTION.
NOTES ON BUILDING CONSTRUCTION. Arranged to
meet the requirements of the syllabus of the Science and Art Department of the
Committee of Council on Education, South Kensington. Medium 8vo.
Part I. First Stage, or Elementary Course. With 552 Woodcuts,
10s. 6d.
Part II. Commencement of Second Stage, or Advanced Course.
With 479 Woodcuts, 10s. 6d.
Part III. Materials. Advanced Course, and Course for Honours.
With 188 Woodcuts, 21s.
Part IV. Calculations for Building Structures. Course for
Honours. With 597 Woodcuts, 15s.
[11]
ELECTRICITY AND MAGNETISM.
CUMMING.—ELECTRICITY TREATED EXPERIMENTALLY.
For the Use of Schools and Students. By Linnæus Cumming,
M.A., Assistant Master in Rugby School. With 242 Illustrations. Crown
8vo., 4s. 6d.
DAY.—EXERCISES IN ELECTRICAL AND MAGNETIC
MEASUREMENTS, with Answers. By R. E. Day. 12mo., 3s. 6d.
DU BOIS.—THE MAGNETIC CIRCUIT IN THEORY
AND PRACTICE. By Dr. H. Du Bois, Privatdocent in the University of
Berlin. Translated by E. Atkinson, Ph.D., formerly Professor of Experimental
Science in the Staff College, Sandhurst. With 94 Illustrations. 8vo.,
12s. net.
GORE.—THE ART OF ELECTRO-METALLURGY, including
all known Processes of Electro-Deposition. By G. Gore, LL.D., F.R.S. With
56 Woodcuts. Fcp. 8vo., 6s.
JENKIN.—ELECTRICITY AND MAGNETISM. By Fleeming
Jenkin, F.R.S.S., L. and E., M.I.C.E. With 177 Illustrations. Fcp. 8vo.,
3s. 6d.
JOUBERT.—ELEMENTARY TREATISE ON ELECTRICITY
AND MAGNETISM. Founded on Joubert's ‘Traité Élémentaire d'Électricité’.
By G. C. Foster, F.R.S., Quain Professor of Physics in University
College, London; and E. Atkinson, Ph.D., formerly Professor of Experimental
Science in the Staff College, Sandhurst. With 381 Illustrations. Crown
8vo., 7s. 6d.
LARDEN.—ELECTRICITY FOR PUBLIC SCHOOLS AND
COLLEGES. By W. Larden, M.A. With 215 Illustrations, and a Series
of Examination Papers, with Answers. Crown 8vo., 6s.
MERRIFIELD.—MAGNETISM AND DEVIATION OF THE
COMPASS. For the Use of Students in Navigation and Science Schools. By
John Merrifield, L.L.D., F.R.A.S., 18mo., 2s. 6d.
POYSER.—Works by A. W. POYSER, M.A., Grammar School,
Wisbeck.
MAGNETISM AND ELECTRICITY. With 235 Illustrations.
Crown 8vo., 2s. 6d.
ADVANCED ELECTRICITY AND MAGNETISM. With
317 Illustrations. Crown 8vo., 4s. 6d.
SLINGO AND BROOKER.—Works by W. SLINGO and A.
BROOKER.
ELECTRICAL ENGINEERING FOR ELECTRIC LIGHT
ARTISANS AND STUDENTS. With 346 Illustrations. Crown 8vo.,
12s.
PROBLEMS AND SOLUTIONS IN ELEMENTARY
ELECTRICITY AND MAGNETISM. Embracing a Complete Set of
Answers to the South Kensington Papers for the years 1885–1894, and a Series
of Original Questions. With 67 Original Illustrations. Crown 8vo., 2s.
TYNDALL.—Works by JOHN TYNDALL, D.C.L., F.R.S. See
p. 27.
[12]
TELEGRAPHY AND THE TELEPHONE.
BENNETT.—THE TELEPHONE SYSTEMS OF CONTINENTAL
EUROPE. By A. R. Bennett, Member of the Institute of
Electrical Engineers; late General Manager in Scotland of the National Telephone
Company, and General Manager and Electrician of the Mutual and New
Telephone Companies. With 169 Illustrations. Crown 8vo., 15s.
CULLEY.—A HANDBOOK OF PRACTICAL TELEGRAPHY.
By R. S. Culley, M.I.C.E., late Engineer-in-Chief of Telegraphs to the Post
Office. With 135 Woodcuts and 17 Plates. 8vo., 16s.
PREECE AND SIVEWRIGHT.—TELEGRAPHY. By W. H.
Preece, C.B., F.R.S., V.P.Inst., C.E., etc., Engineer-in-Chief and Electrician
Post Office Telegraphs; and Sir J. Sivewright, K.C.M.G., General Manager,
South African Telegraphs. With 258 Woodcuts. Fcp. 8vo., 6s.
ENGINEERING, STRENGTH OF MATERIALS, ETC.
ANDERSON.—THE STRENGTH OF MATERIALS AND
STRUCTURES: the Strength of Materials as depending on their Quality and
as ascertained by Testing Apparatus. By Sir J. Anderson, C.E., LL.D.,
F.R.S.E. With 66 Woodcuts. Fcp. 8vo., 3s. 6d.
BARRY.—RAILWAY APPLIANCES: a Description of Details
of Railway Construction subsequent to the completion of the Earthworks and
Structures. By John Wolfe Barry, C.B., M.I.C.E. With 218 Woodcuts.
Fcp. 8vo., 4s. 6d.
SMITH.—GRAPHICS, or the Art of Calculation by Drawing
Lines, applied especially to Mechanical Engineering. By Robert H. Smith,
Professor of Engineering, Mason College, Birmingham. Part I. With separate
Atlas of 29 Plates containing 97 Diagrams. 8vo., 15s.
STONEY.—THE THEORY OF THE STRESSES ON
GIRDERS AND SIMILAR STRUCTURES. With Practical Observations on
the Strength and other Properties of Materials. By Bindon B. Stoney,
LL.D., F.R.S., M.I.C.E. With 5 Plates and 143 Illustrations in the Text.
Royal 8vo., 36s.
UNWIN.—Works by WILLIAM CAWTHORNE UNWIN,
F.R.S., B.S.C.
THE TESTING OF MATERIALS OF CONSTRUCTION.
Embracing the description of Testing Machinery and Apparatus Auxiliary
to Mechanical Testing, and an Account of the most Important Researches
on the Strength of Materials. With 141 Woodcuts and 5 Folding-out Plates.
8vo., 21s.
ON THE DEVELOPMENT AND TRANSMISSION OF
POWER FROM CENTRAL STATIONS: being the Howard Lectures
delivered at the Society of Arts in 1893. With 81 Diagrams. 8vo.,
10s. net.
WARREN.—ENGINEERING CONSTRUCTION IN IRON,
STEEL, AND TIMBER. By William Henry Warren, Challis Professor
of Civil and Mechanical Engineering, University of Sydney. With 13 Folding
Plates, and 375 Diagrams. Royal 8vo., 16s. net.
[13]
MACHINE DRAWING AND DESIGN.
LOW AND BEVIS.—A MANUAL OF MACHINE DRAWING
AND DESIGN. By David Allan Low (Whitworth Scholar), M.I.Mech.E.,
Head Master of the Technical School, People's Palace, London; and Alfred
William Bevis (Whitworth Scholar), M.I.Mech.E., Director of Manual
Training to the Birmingham School Board. With over 700 Illustrations. 8vo.,
7s. 6d.
LOW.—Works by DAVID ALLAN LOW, Head Master of the
Technical School, People's Palace, London.
IMPROVED DRAWING SCALES. 4d. in case.
AN INTRODUCTION TO MACHINE DRAWING AND
DESIGN. With 97 Illustrations and Diagrams. Crown 8vo., 2s.
UNWIN.—THE ELEMENTS OF MACHINE DESIGN. By
W. Cawthorne Unwin, F.R.S., Professor of Engineering at the Central
Institute of the City and Guilds of London Institute.
Part I. General Principles, Fastenings, and Transmissive
Machinery. With 304 Diagrams, etc. Crown 8vo., 6s.
Part II. Chiefly on Engine Details. With 174 Woodcuts.
Crown 8vo., 4s. 6d.
LONGMANS' CIVIL ENGINEERING SERIES.
Edited by the Author of ‘Notes on Building Construction’.
TIDAL RIVERS: their (1) Hydraulics, (2) Improvement, (3)
Navigation. By W. H. Wheeler, M.Inst.C.E., author of ‘The Drainage of
Fens and Low Lands by Gravitation and Steam Power’. With 75 Illustrations.
Medium 8vo., 16s. net.
NOTES ON DOCKS AND DOCK CONSTRUCTION. By C.
Colson, M.Inst.C.E., Assistant Director of Works, Admiralty. With 365
Illustrations. Medium 8vo., 21s. net.
PRINCIPLES AND PRACTICE OF HARBOUR CONSTRUCTION.
By William Shield, F.R.S.E., M.Inst.C.E., and Executive
Engineer, National Harbour of Refuge, Peterhead, N.B. With 97 Illustrations.
Medium 8vo., 15s. net.
CALCULATIONS FOR ENGINEERING STRUCTURES. By
T. Claxton Fidler, M.I.C.E., Professor of Engineering in the University of
Dundee; Author of ‘A Practical Treatise on Bridge Construction’. [In preparation.
A COURSE OF CIVIL ENGINEERING. By L. F. Vernon-Harcourt,
M.Inst.C.E., Professor of Civil Engineering at University College,
London. [In preparation.
RAILWAY CONSTRUCTION. By W. H. MILLS, M.I.C.E.,
Engineer-in-Chief, Great Northern Railway, Ireland. [In preparation.
[14]
WORKSHOP APPLIANCES, ETC.
NORTHCOTT.—LATHES AND TURNING, Simple, Mechanical
and Ornamental. By W. H. Northcott. With 338 Illustrations.
8vo., 18s.
SHELLEY.—WORKSHOP APPLIANCES, including Descriptions
of some of the Gauging and Measuring Instruments, Hand-cutting Tools,
Lathes, Drilling, Plaining, and other Machine Tools used by Engineers. By
C. P. B. Shelley, M.I.C.E. With an additional Chapter on Milling by R.
R. Lister. With 323 Woodcuts. Fcp. 8vo., 5s.
MINERALOGY, METALLURGY, ETC.
BAUERMAN.—Works by HILARY BAUERMAN, F.G.S.
SYSTEMATIC MINERALOGY. With 373 Woodcuts and Diagrams. Fcp. 8vo., 6s.
DESCRIPTIVE MINERALOGY. With 236 Woodcuts and Diagrams. Fcp. 8vo., 6s.
BLOXAM AND HUNTINGTON.—METALS: their Properties
and Treatment. By C. L. Bloxam and A. K. Huntington, Professors in
King's College, London. With 130 Woodcuts. Fcp. 8vo., 5s.
GORE.—THE ART OF ELECTRO-METALLURGY, including
all known Processes of Electro-Deposition. By G. Gore, LL.D., F.R.S.
With 56 Woodcuts. Fcp. 8vo., 6s.
MITCHELL.—A MANUAL OF PRACTICAL ASSAYING.
By John Mitchell, F.C.S. Revised, with the Recent Discoveries incorporated.
By W. Crookes, F.R.S. With 201 Illustrations. 8vo., 31s. 6d.
RHEAD.—METALLURGY. An Elementary Text-Book. By
E. C. Rhead, Lecturer on Metallurgy at the Municipal Technical School,
Manchester. With 94 Illustrations. Crown 8vo., 3s. 6d.
RUTLEY.—THE STUDY OF ROCKS: an Elementary Text-book
of Petrology. By F. Rutley, F.G.S. With 6 Plates and 88 Woodcuts.
Fcp. 8vo., 4s. 6d.
ASTRONOMY, NAVIGATION, ETC.
ABBOTT.—ELEMENTARY THEORY OF THE TIDES:
the Fundamental Theorems Demonstrated without Mathematics and the Influence
on the Length of the Day Discussed. By T. K. Abbott, B.D., Fellow
and Tutor, Trinity College, Dublin. Crown 8vo., 2s.
BALL.—Works by Sir ROBERT S. BALL, LL.D., F.R.S.
ELEMENTS OF ASTRONOMY. With 130 Figures and Diagrams. Fcp. 8vo., 6s. 6d.
A CLASS-BOOK OF ASTRONOMY. With 41 Diagrams. Fcp. 8vo., 1s. 6d.
[15]
BRINKLEY.—ASTRONOMY. By F. Brinkley, formerly
Astronomer Royal for Ireland. Re-edited and Revised by J. W. Stubbs, D.D.,
and F. Brünnow, Ph.D. With 49 Diagrams. Crown 8vo., 6s.
CLERKE.—THE SYSTEM OF THE STARS. By Agnes M.
Clerke. With 6 Plates, and numerous Illustrations. 8vo., 21s.
GOODWIN.—AZIMUTH TABLES FOR THE HIGHER
DECLINATIONS. (Limits of Declination 24° to 30°, both inclusive.)
Between the Parallels of Latitude 0° and 60°. With Examples of the Use of
the Tables in English and French. By H. B. Goodwin, Naval Instructor,
Royal Navy. Royal 8vo., 7s. 6d.
HERSCHEL.—OUTLINES OF ASTRONOMY.—By Sir John
F. W. Herschel, Bart., K.H., etc. With 9 Plates, and numerous Diagrams.
8vo., 12s.
LOWELL.—MARS. By Percival Lowell, Fellow American
Academy, Member Royal Asiatic Society, Great Britain and Ireland, etc.
With 24 Plates. 8vo., 12s. 6d.
MARTIN.—NAVIGATION AND NAUTICAL ASTRONOMY.
Compiled by Staff Commander W. R. Martin, R.N. Royal 8vo., 18s.
MERRIFIELD.—A TREATISE ON NAVIGATION. For
the Use of Students. By J. Merrifield, LL.D., F.R.A.S., F.M.S. With
Charts and Diagrams. Crown 8vo., 5s.
PARKER.—ELEMENTS OF ASTRONOMY. With Numerous
Examples and Examination Papers. By George W. Parker, M.A., of
Trinity College, Dublin. With 84 Diagrams. 8vo., 5s. net.
WEBB.—CELESTIAL OBJECTS FOR COMMON TELESCOPES.
By the Rev. T. W. Webb, M.A., F.R.A.S. Fifth Edition,
Revised and greatly Enlarged by the Rev. T. E. Espin, M.A., F.R.A.S. (Two
Volumes.) Vol. I., with Portrait and a Reminiscence of the Author, 2 Plates,
and numerous Illustrations. Crown 8vo., 6s. Vol. II., with numerous Illustrations.
Crown 8vo., 6s. 6d.
WORKS BY RICHARD A. PROCTOR.
OLD AND NEW ASTRONOMY. With 21 Plates and 472
Illustrations in the Text. 4to., 21s.
MYTHS AND MARVELS OF ASTRONOMY. Crown 8vo., 3s. 6d.
THE MOON: Her Motions, Aspect, Scenery, and Physical
Condition. With many Plates and Charts, Wood Engravings, and 2 Lunar
Photographs. Crown 8vo., 5s.
THE UNIVERSE OF STARS: Researches into, and New Views
respecting the Constitution of the Heavens. With 22 Charts (4 Coloured), and
22 Diagrams. 8vo., 10s. 6d.
[16]
OTHER WORLDS THAN OURS: the Plurality of Worlds
Studied Under the Light of Recent Scientific Researches. With 14 Illustrations;
Map, Charts, etc. Crown 8vo., 3s. 6d.
THE ORBS AROUND US; Essays on the Moon and Planets,
Meteors and Comets, the Sun and Coloured Pairs of Suns. Crown 8vo., 3s. 6d.
LIGHT SCIENCE FOR LEISURE HOURS: Familiar Essays
on Scientific Subjects. Natural Phenomena, etc. 3 vols., crown 8vo., 5s. each.
THE EXPANSE OF HEAVEN: Essays on the Wonders of the
Firmament. Crown 8vo., 3s. 6d.
OTHER SUNS THAN OURS: a Series of Essays on Suns—Old,
Young, and Dead. With other Science Gleanings. Two Essays on Whist,
and Correspondence with Sir John Herschel. With 9 Star-Maps and Diagrams.
Crown 8vo., 3s. 6d.
HALF-HOURS WITH THE TELESCOPE: a Popular Guide
to the Use of the Telescope as a means of Amusement and Instruction. With
7 Plates. Fcp. 8vo., 2s. 6d.
NEW STAR ATLAS FOR THE LIBRARY, the School, and
the Observatory, in Twelve Circular Maps (with Two Index-Plates). With an
Introduction on the Study of the Stars. Illustrated by 9 Diagrams. Crown 8vo.,
5s.
THE SOUTHERN SKIES: a Plain and Easy Guide to the
Constellations of the Southern Hemisphere. Showing in 12 Maps the position
of the principal Star-Groups night after night throughout the year. With an
Introduction and a separate Explanation of each Map. True for every Year.
4to., 5s.
HALF-HOURS WITH THE STARS: a Plain and Easy Guide
to the Knowledge of the Constellations. Showing in 12 Maps the position of
the principal Star-Groups night after night throughout the year. With Introduction
and a separate Explanation of each Map. True for every Year.
4to., 3s. 6d.
LARGER STAR ATLAS FOR OBSERVERS AND STUDENTS.
In Twelve Circular Maps, showing 6000 Stars, 1500 Double Stars, Nebulæ, etc.
With 2 Index-Plates. Folio, 15s.
THE STARS IN THEIR SEASONS: an Easy Guide to a
Knowledge of the Star-Groups. In 12 Large Maps. Imperial 8vo., 5s.
ROUGH WAYS MADE SMOOTH. Familiar Essays on
Scientific Subjects. Crown 8vo., 3s. 6d.
PLEASANT WAYS IN SCIENCE. Crown 8vo., 3s. 6d.
NATURE STUDIES. By R. A. Proctor, Grant Allen, A.
Wilson, T. Foster, and E. Clodd. Crown 8vo., 3s. 6d.
LEISURE READINGS. By R. A. Proctor, E. Clodd, A.
Wilson, T. Foster, and A. C. Ranyard. Crown 8vo., 3s. 6d.
[17]
MANUFACTURES, TECHNOLOGY, ETC.
BELL.—JACQUARD WEAVING AND DESIGNING. By F. T.
Bell, Medallist in Honours and Certificated Teacher in ‘Linen Manufacturing’
and in ‘Weaving and Pattern Designing,’ City and Guilds of London Institute.
With 199 Diagrams. 8vo., 12s. net.
CROSS AND BEVAN.—CELLULOSE: an Outline of the
Chemistry of the Structural Elements of Plants. With Reference to their
Natural History and Industrial Uses. By Cross and Bevan (C. F. Cross, E.
J. Bevan, and C. Beadle). With 14 Plates. Crown 8vo., 12s. net.
TAYLOR.—COTTON WEAVING AND DESIGNING. By
John T. Taylor. With 373 Diagrams. Crown 8vo., 7s. 6d. net.
LUPTON.—MINING. An Elementary Treatise on the Getting
of Minerals. By Arnold Lupton, M.I.C.E., F.G.S., etc. With 596 Diagrams
and Illustrations. Crown 8vo., 9s. net.
WATTS.—AN INTRODUCTORY MANUAL FOR SUGAR
GROWERS. By Francis Watts, F.C.S., F.I.C. With 20 Illustrations.
Crown 8vo., 6s.
PHYSIOGRAPHY AND GEOLOGY.
BIRD.—Works by CHARLES BIRD, B.A.
ELEMENTARY GEOLOGY. With Geological Map of the
British Isles, and 247 Illustrations. Crown 8vo., 2s. 6d.
ADVANCED GEOLOGY. A Manual for Students in Advanced
Classes and for General Readers. With over 300 Illustrations, a Geological
Map of the British Isles (coloured), and a set of Questions for Examination.
Crown 8vo., 7s. 6d.
GREEN.—PHYSICAL GEOLOGY FOR STUDENTS AND
GENERAL READERS. With Illustrations. By A. H. Green, M.A.,
F.G.S. 8vo., 21s.
THORNTON.—Works by J. THORNTON, M.A.
ELEMENTARY PHYSIOGRAPHY: an Introduction to the
Study of Nature. With 12 Maps and 247 Illustrations. With Appendix on
Astronomical Instruments and Measurements. Crown 8vo., 2s. 6d.
ADVANCED PHYSIOGRAPHY. With 6 Maps and 180
Illustrations. Crown 8vo., 4s. 6d.
HEALTH AND HYGIENE.
BRAY.—PHYSIOLOGY AND THE LAWS OF HEALTH, in,
Easy Lessons for Schools. By Mrs. Charles Bray. Fcp. 8vo., 1s.
BRODRIBB.—MANUAL OF HEALTH AND TEMPERANCE.
By T. Brodribb, M.A. With Extracts from Gough's ‘Temperance
Orations’. Revised and Edited by the Rev. W. Ruthven Pym, M.A., Member
of the Sheffield School Board. Crown 8vo., 1s. 6d.
BUCKTON.—HEALTH IN THE HOUSE; Twenty-five
Lectures on Elementary Physiology. By Mrs. C. M. Buckton. With 41
Woodcuts and Diagrams. Crown 8vo., 2s.
[18]
CORFIELD.—THE LAWS OF HEALTH. By W. H. Corfield,
M.A., M.D. Fcp. 8vo., 1s. 6d.
FRANKLAND.—MICRO-ORGANISMS IN WATER, THEIR
SIGNIFICANCE, IDENTIFICATION, AND REMOVAL. Together with
an Account of the Bacteriological Methods Involved in their Investigation.
Specially Designed for the Use of those connected with the Sanitary Aspects of
Water Supply. By Professor Percy Frankland, Ph.D., B.Sc. (Lond.),
F.R.S., Fellow of the Chemical Society; and Mrs. Percy Frankland. With
2 Plates and numerous Diagrams. 8vo., 16s. net.
NOTTER AND FIRTH.—HYGIENE. By J. L. Notter, M.A.,
M.D., and R. H. Firth, F.R.C.S. With 95 Illustrations. Crown 8vo., 3s. 6d.
POORE.—ESSAYS ON RURAL HYGIENE. By George
Vivian Poore, M.D. Crown 8vo., 6s. 6d.
WILSON.—A MANUAL OF HEALTH-SCIENCE: adapted
for use in Schools and Colleges, and suited to the requirements of Students
preparing for the Examinations in Hygiene of the Science and Art Department,
etc. By Andrew Wilson, F.R.S.E., F.L.S., etc. With 74 Illustrations.
Crown 8vo., 2s. 6d.
NATURAL HISTORY, EVOLUTION, ETC.
CLODD.—Works by EDWARD CLODD.
THE STORY OF CREATION: A Plain Account of Evolution.
With 77 Illustrations. Crown 8vo., 3s. 6d.
A PRIMER OF EVOLUTION: being a Popular Abridged
Edition of ‘The Story of Creation’. With Illustrations. Fcp. 8vo., 1s. 6d.
FURNEAUX.—Works by WILLIAM S. FURNEAUX.
THE OUTDOOR WORLD; or, The Young Collector's Handbook.
With 18 Plates, 16 of which are coloured, and 549 Illustrations in the
Text. Crown 8vo., 7s. 6d.
BUTTERFLIES AND MOTHS (British). With 12 Coloured
Plates and 241 Illustrations in the Text. 12s. 6d.
LIFE IN PONDS AND STREAMS. With 8 Coloured Plates
and 311 Illustrations in the Text. Crown 8vo., 12s. 6d.
HUDSON.—BRITISH BIRDS. By W. H. Hudson, C.M.Z.S.
With 8 Coloured Plates from Original Drawings by A. Thorburn, and 8 Plates
and 100 Figures in black and white from Original Drawings by C. E. Lodge,
and 3 Illustrations from Photographs by R. B. Lodge. Crown 8vo., 12s. 6d.
ROMANES.—Works by GEORGE JOHN ROMANES, LL.D.,
F.R.S.
DARWIN, AND AFTER DARWIN: an Exposition of the
Darwinian Theory, and a Discussion on Post-Darwinian Questions.
Part I. THE DARWINIAN THEORY. With Portrait of Darwin and 125
Illustrations. Crown 8vo., 10s. 6d.
Part II. POST-DARWINIAN QUESTIONS: Heredity and Utility. With
Portrait of the Author and 5 Illustrations. Crown 8vo., 10s. 6d.
AN EXAMINATION OF WEISMANNISM. Crown 8vo., 6s.
[19]
MEDICINE AND SURGERY.
ASHBY AND WRIGHT.—THE DISEASES OF CHILDREN,
MEDICAL AND SURGICAL. By Henry Ashby, M.D., Lond., F.R.C.P.,
and G. A. Wright, B.A., M.B., Oxon., F.R.C.S., Eng. With 192 Illustrations.
8vo., 25s.
BENNETT.—Works by WILLIAM H. BENNETT, F.R.C.S.,
Surgeon to St. George's Hospital.
CLINICAL LECTURES ON VARICOSE VEINS OF THE
LOWER EXTREMITIES. With 3 Plates. 8vo., 6s.
ON VARICOCELE; A PRACTICAL TREATISE. With 4
Tables and a Diagram. 8vo., 5s.
CLINICAL LECTURES ON ABDOMINAL HERNIA:
chiefly in relation to Treatment, including the Radical Cure. With 12 Diagrams
in the Text. 8vo., 8s. 6d.
CLARKE.—POST-MORTEM EXAMINATIONS IN
MEDICO-LEGAL AND ORDINARY CASES. With Special Chapters on
the Legal Aspects of Post-Mortems, and on Certificates of Death. By J.
Jackson Clarke, M.B. (Lond.), F.R.C.S. Fcp. 8vo., 2s. 6d.
COATS.—A MANUAL OF PATHOLOGY. By Joseph
Coats, M.D., Professor of Pathology in the University of Glasgow. Third
Edition. Revised throughout. With 507 Illustrations. 8vo., 31s. 6d.
COOKE.—Works by THOMAS COOKE, F.R.C.S. Eng., B.A.,
B.Sc., M.D., Paris.
TABLETS OF ANATOMY. Being a Synopsis of Demonstrations
given in the Westminster Hospital Medical School in the years 1871–75.
Tenth Thousand, being a selection of the Tablets believed to be most useful
to Students generally. Post 4to., 10s. 6d.
APHORISMS IN APPLIED ANATOMY AND OPERATIVE
SURGERY. Including 100 Typical viva voce Questions on Surface Marking,
etc. Crown 8vo., 3s. 6d.
DISSECTION GUIDES. Aiming at Extending and Facilitating
such Practical work in Anatomy as will be specially useful in connection with
an ordinary Hospital Curriculum. 8vo., 10s. 6d.
DICKINSON.—Works by W. HOWSHIP DICKINSON, M.D.
Cantab., F.R.C.P.
ON RENAL AND URINARY AFFECTIONS. Complete
in Three Parts, 8vo., with 12 Plates and 122 Woodcuts. Price £3 4s. 6d.
cloth.
∵ The Parts can also be had separately, each complete in itself, as follows:—
Part I. Diabetes, 10s. 6d. sewed, 12s. cloth.
Part II. Albuminuria, £1 sewed, £1 1s. cloth.
Part III. Miscellaneous Affections of the Kidneys and Urine,
£1 10s. sewed, £1 11s. 6d. cloth.
[20]
THE TONGUE AS AN INDICATION OF DISEASE;
being the Lumleian Lectures delivered at the Royal College of Physicians in
March, 1888. 8vo., 7s. 6d.
THE HARVEIAN ORATION ON HARVEY IN ANCIENT
AND MODERN MEDICINE. Crown 8vo., 2s. 6d.
OCCASIONAL PAPERS ON MEDICAL SUBJECTS, 1855–1896.
8vo., 12s.
ERICHSEN.—THE SCIENCE AND ART OF SURGERY;
a Treatise on Surgical Injuries, Diseases, and Operations. Tenth Edition. Revised
by the late Marcus Beck, M.S., and M.B. (Lond.), F.R.C.S., and by
Raymond Johnson, M.B. and B.S. (Lond.), F.R.C.S. Illustrated by nearly
1000 Engravings on Wood. 2 vols., royal 8vo., 28s.
GARROD.—Works by Sir ALFRED BARING GARROD,
M.D., F.R.S., etc.
A TREATISE ON GOUT AND RHEUMATIC GOUT
(RHEUMATOID ARTHRITIS). With 6 Plates, comprising 21 Figures
(14 Coloured), and 27 Illustrations engraved on Wood. 8vo., 21s.
THE ESSENTIALS OF MATERIA MEDICA AND THERAPEUTICS.
Revised and Edited, under the supervision of the Author, by
Nestor Tirard, M.D., Lond., F.R.C.P., Professor of Materia Medica and
Therapeutics in King's College, London, etc. Crown 8vo., 12s. 6d.
GRAY.—ANATOMY, DESCRIPTIVE AND SURGICAL. By
Henry Gray, F.R.S., late Lecturer on Anatomy at St. George's Hospital.
The Thirteenth Edition, re-edited by T. Pickering Pick, Surgeon to St.
George's Hospital; Member of the Court of Examiners, Royal College of
Surgeons of England. With 636 large Woodcut Illustrations, a large proportion
of which are coloured, the Arteries being coloured red, the Veins blue, and
the Nerves yellow. The attachments of the muscles to the bones, in the section
on Osteology, are also shown in coloured outline. Royal 8vo., 36s.
HALLIBURTON.—Works by W. D. HALLIBURTON, M.D.,
F.R.S., M.R.C.P.
A TEXT-BOOK OF CHEMICAL PHYSIOLOGY AND
PATHOLOGY. With 104 Illustrations. 8vo. 28s.
ESSENTIALS OF CHEMICAL PHYSIOLOGY. Second
Edition. 8vo., 5s.
LANG.—THE METHODICAL EXAMINATION OF THE
EYE. Being Part I. of a Guide to the Practice of Ophthalmology for Students
and Practitioners. By William Lang, F.R.C.S., Eng., Surgeon to the Royal
London Ophthalmic Hospital, Moorfields, etc. With 15 Illustrations.
Crown 8vo., 3s. 6d.
[21]
LIVEING.—Works by ROBERT LIVEING, M.A. and M.D.
Cantab., F.R.C.P., Lond., etc., Physician to the Department
for Diseases of the Skin at the Middlesex Hospital, etc.
HANDBOOK ON DISEASES OF THE SKIN. With especial
reference to Diagnosis and Treatment. Fcap. 8vo., 5s.
ELEPHANTIASIS GRÆCORUM, OR TRUE LEPROSY;
Being the Goulstonian Lectures for 1873. Cr. 8vo., 4s. 6d.
LONGMORE.—Works by Surgeon-General Sir T. LONGMORE
(Retired), C.B., F.R.C.S.
THE ILLUSTRATED OPTICAL MANUAL; OR, HANDBOOK
OF INSTRUCTIONS FOR THE GUIDANCE OF SURGEONS
IN TESTING QUALITY AND RANGE OF VISION, and in Distinguishing
and Dealing with Optical Defects in General.
Illustrated by 74 Drawings and Diagrams by Inspector-General Dr.
Macdonald, R.N., F.R.S., C.B. 8vo., 14s.
GUNSHOT INJURIES. Their History, Characteristic Features,
Complications, and General Treatment; with Statistics concerning them
as they have been met with in Warfare. With 78 Illustrations. 8vo.,
31s. 6d.
LUFF.—TEXT-BOOK OF FORENSIC MEDICINE AND
TOXICOLOGY. By Arthur P. Luff, M.D., B.Sc. (Lond.), Physician
in Charge of Out-Patients and Lecturer on Medical Jurisprudence and
Toxicology in St. Mary's Hospital; Examiner in Forensic Medicine in the
University of London; External Examiner in Forensic Medicine in the
Victoria University; Official Analyst to the Home Office. With numerous
Illustrations. 2 vols., crown 8vo., 24s.
NEWMAN.—ON THE DISEASES OF THE KIDNEY
AMENABLE TO SURGICAL TREATMENT. Lectures to Practitioners.
By David Newman, M.D., Surgeon to the Western Infirmary Out-Door
Department; Pathologist and Lecturer on Pathology at the Glasgow Royal
Infirmary; Examiner in Pathology in the University of Glasgow; Vice-President
Glasgow Pathological and Clinical Society. 8vo., 16s.
OWEN.—A MANUAL OF ANATOMY FOR SENIOR
STUDENTS. By Edmund Owen, M.B., F.R.S.C., Senior Surgeon to the
Hospital for Sick Children, Great Ormond Street, Surgeon to St. Mary's
Hospital, London, and co-Lecturer on Surgery, late Lecturer on Anatomy in
its Medical School. With 210 Illustrations. Crown 8vo., 12s. 6d.
POOLE.—COOKERY FOR THE DIABETIC. By W. H. and
Mrs. Poole. With Preface by Dr. Pavy. Fcap. 8vo., 2s. 6d.
[22]
QUAIN.—A DICTIONARY OF MEDICINE; Including
General Pathology, General Therapeutics, Hygiene, and the Diseases of
Women and Children. By Various Writers. Edited by Richard Quain,
Bart., M.D. Lond., LL.D. Edin., (Hon.) F.R.S., Physician Extraordinary to
H.M. the Queen, President of the General Medical Council, Member of the
Senate of the University of London, etc. Assisted by Frederick Thomas
Roberts, M.D. Lond., B.Sc., Fellow of the Royal College of Physicians,
Fellow of University College, Professor of Materia Medica and Therapeutics,
University College, &c.; and J. Mitchell Bruce, M.A. Abdn., M.D. Lond.,
Fellow of the Royal College of Physicians of London, Physician and Lecturer
on the Principles and Practice of Medicine, Charing Cross Hospital, &c. New
Edition, Revised throughout and Enlarged. In 2 Vols., medium 8vo., cloth,
red edges, price 40s. net.
QUAIN.—QUAIN'S (JONES) ELEMENTS OF ANATOMY.
The Tenth Edition. Edited by Edward Albert Schäfer, F.R.S., Professor
of Physiology and Histology in University College, London; and George
Dancer Thane, Professor of Anatomy in University College, London. In 3
Vols.
∵ The several parts of this work form complete Text-books of their respective
subjects. They can be obtained separately as follows:—
Vol. I., Part I. EMBRYOLOGY.
By E. A. Schäfer, F.R.S. With
200 Illustrations. Royal 8vo., 9s.
Vol. I., Part II. GENERAL ANATOMY
OR HISTOLOGY. By E.
A. Schäfer, F.R.S. With 291
Illustrations. Royal 8vo., 12s. 6d.
Vol. II., Part I. OSTEOLOGY. By
G. D. Thane. With 168 Illustrations.
Royal 8vo., 9s.
Vol. II., Part II. ARTHROLOGY—MYOLOGY—ANGEIOLOGY.
By G. D. Thane. With 255 Illustrations.
Royal 8vo., 18s.
Vol. III., Part I. THE SPINAL
CORD AND BRAIN. By E. A.
Schäfer, F.R.S. With 139 Illustrations.
Royal 8vo., 12s. 6d.
Vol. III., Part II. THE NERVES.
By G. D. Thane. With 102
Illustrations. Royal 8vo., 9s.
Vol. III., Part III. THE ORGANS
OF THE SENSES. By E. A.
Schäfer, F.R.S. With 178 Illustrations.
Royal 8vo., 9s.
Vol. III., Part IV. SPLANCHNOLOGY.
By E. A. Schäfer,
F.R.S., and Johnson Symington,
M.D. With 337 Illustrations. Royal
8vo., 16s.
Appendix. SUPERFICIAL AND
SURGICAL ANATOMY. By
Professor G. D. Thane and Professor
R. J. Godlee, M.S. With
29 Illustrations. Royal 8vo., 6s. 6d.
SCHÄFER.—THE ESSENTIALS OF HISTOLOGY. Descriptive
and Practical. For the Use of Students. By E. A. Schäfer, F.R.S.,
Jodrell Professor of Physiology in University College, London; Editor of the
Histological Portion of Quain's ‘Anatomy’. Illustrated by more than 300
Figures, many of which are new. 8vo., 7s. 6d. (Interleaved, 10s.)
SCHENK.—MANUAL OF BACTERIOLOGY. For Practitioners
and Students. With especial reference to Practical Methods. By Dr.
S. L. Schenk, Professor (Extraordinary) in the University of Vienna. Translated
from the German, with an Appendix, by W. R. Dawson, B.A., M.D.,
Univ. Dub.; late University Travelling Prizeman in Medicine. With 100
Illustrations, some of which are coloured. 8vo., 10s. net.
[23]
SMALE AND COLYER. DISEASES AND INJURIES OF
THE TEETH, including Pathology and Treatment: a Manual of Practical
Dentistry for Students and Practitioners. By MORTON SMALE M.R.C.S.,
L.S.A., L.D.S., Dental Surgeon to St. Mary's Hospital, Dean of the School,
Dental Hospital of London, etc.; and J. F. COLYER, L.R.C.P., M.R.C.S.,
L.D.S., Assistant Dental Surgeon to Charing Cross Hospital, and Assistant
Dental Surgeon to the Dental Hospital of London. With 334 Illustrations.
Large Crown 8vo., 15s.
SMITH (H. F.). THE HANDBOOK FOR MIDWIVES. By
HENRY FLY SMITH, B.A., M.B. Oxon., M.R.C.S. With 41 Woodcuts.
Crown 8vo., price 5s.
STEEL.—Works by JOHN HENRY STEEL, F.R.C.V.S., F.Z.S.,
A.V.D.
A TREATISE ON THE DISEASES OF THE DOG; being
a Manual of Canine Pathology. Especially adapted for the use of Veterinary
Practitioners and Students. 88 Illustrations. 8vo., 10s. 6d.
A TREATISE ON THE DISEASES OF THE OX; being a
Manual of Bovine Pathology. Especially adapted for the use of Veterinary
Practitioners and Students. 2 Plates and 117 Woodcuts. 8vo., 15s.
A TREATISE ON THE DISEASES OF THE SHEEP; being
a Manual of Ovine Pathology for the use of Veterinary Practitioners and
Students. With Coloured Plate and 99 Woodcuts. 8vo., 12s.
OUTLINES OF EQUINE ANATOMY; a Manual for the use
of Veterinary Students in the Dissecting Room. Crown 8vo., 7s. 6d.
‘STONEHENGE.’—THE DOG IN HEALTH AND DISEASE.
By ‘Stonehenge’. With 84 Wood Engravings. Square Crown
8vo., 7s. 6d.
WALLER.—AN INTRODUCTION TO HUMAN PHYSIOLOGY.
By AUGUSTUS D. WALLER, M.D., Lecturer on Physiology at St.
Mary's Hospital Medical School, London; late External Examiner at the
Victorian University. Second Edition, Revised. With 305 Illustrations. 8vo.,
18s.
WEICHSELBAUM.—THE ELEMENTS OF PATHOLOGICAL
HISTOLOGY. With Special Reference to Practical Methods. By Dr.
Anton Weichselbaum, Professor of Pathology in the University of Vienna.
Translated by W. R. Dawson, M.D. (Dub.), Demonstrator of Pathology in
the Royal College of Surgeons, Ireland, late Medical Travelling Prizeman of
Dublin University, &c. With 221 Figures, partly in Colours, a Cromo-lithographic
Plate, and 7 Photographic Plates. Royal 8vo., 21s. net.
[24]
WILKS AND MOXON.—LECTURES ON PATHOLOGICAL
ANATOMY. By Samuel Wilks, M.D., F.R.S., Consulting Physician to,
and formerly Lecturer on Medicine and Pathology at Guy's Hospital, and the
late Walter Moxon, M.D., F.R.C.P., Physician to, and some time Lecturer
on Pathology at Guy's Hospital. Third Edition, thoroughly Revised. By
Samuel Wilks, M.D., LL.D., F.R.S. 8vo., 18s.
YOUATT.—Works by WILLIAM YOUATT.
THE HORSE. Revised and Enlarged by W. Watson,
M.R.C.V.S. With 52 Woodcuts. 8vo., 7s. 6d.
THE DOG. Revised and Enlarged. With 33 Woodcuts. 8vo., 6s.
PHYSIOLOGY, BIOLOGY, ETC.
ASHBY.—NOTES ON PHYSIOLOGY, for the Use of Students
Preparing for Examination. By Henry Ashby, M.D. With 141 Illustrations.
Fcp. 8vo., 5s.
BARNETT.—THE MAKING OF THE BODY: a Children's
Book on Anatomy and Physiology, for School and Home Use. By Mrs. S. A.
Barnett. With 113 Illustrations. Crown 8vo., 1s. 9d.
BIDGOOD.—A COURSE OF PRACTICAL ELEMENTARY
BIOLOGY. By John Bidgood, B.Sc., F.L.S. With 226 Illustrations.
Crown 8vo., 4s. 6d.
BRAY.—PHYSIOLOGY AND THE LAWS OF HEALTH, in
Easy Lessons for Schools. By Mrs. Charles Bray. Fcp. 8vo., 1s.
FRANKLAND.—MICRO-ORGANISMS IN WATER. Together
with an Account of the Bacteriological Methods involved in their
Investigation. Specially designed for the use of those connected with the
Sanitary Aspects of Water-Supply. By Percy Frankland, Ph.D., B.Sc.
(Lond.), F.R.S., and Mrs. Percy Frankland. With 2 Plates and Numerous
Diagrams. 8vo., 16s. net.
FURNEAUX.—HUMAN PHYSIOLOGY. By W. Furneaux,
F.R.G.S. With 218 Illustrations. Crown 8vo., 2s. 6d.
HUDSON AND GOSSE.—THE ROTIFERA, or ‘WHEEL-ANIMALCULES’.
By C. T. Hudson, LL.D., and P. H. Gosse, F.R.S.
With 30 Coloured and 4 Uncoloured Plates. In 6 Parts. 4to., 10s. 6d. each;
Supplement 12s. 6d. Complete in 2 vols., with Supplement, 4to., £4 4s.
[25]
MACALISTER.—Works by ALEXANDER MACALISTER,
M.D.
ZOOLOGY AND MORPHOLOGY OF VERTEBRATA. 8vo., 10s. 6d.
ZOOLOGY OF THE INVERTEBRATE ANIMALS. With 59 Diagrams. Fcp. 8vo., 1s. 6d.
ZOOLOGY OF THE VERTEBRATE ANIMALS. With 77 Diagrams. Fcp. 8vo., 1s. 6d.
MORGAN.—ANIMAL BIOLOGY: an Elementary Text-Book.
By C. Lloyd Morgan. With numerous Illustrations. Crown 8vo., 8s. 6d.
SCHENK.—MANUAL OF BACTERIOLOGY, for Practitioners
and Students, with Especial Reference to Practical Methods. By Dr. S. L.
Schenk. With 100 Illustrations, some of which are Coloured. 8vo., 10s. net.
THORNTON.—HUMAN PHYSIOLOGY. By John Thornton,
M.A. With 267 Illustrations, some Coloured. Crown 8vo., 6s.
BOTANY.
AITKEN.—ELEMENTARY TEXT-BOOK OF BOTANY.
For the use of Schools. By Edith Aitken, late Scholar of Girton College.
With over 400 Diagrams. Crown 8vo., 4s. 6d.
BENNETT AND MURRAY.—HANDBOOK OF CRYPTOGAMIC
BOTANY. By Alfred W. Bennett, M.A., B.Sc., F.L.S., Lecturer
on Botany at St. Thomas's Hospital; and George Murray, F.L.S., Keeper
of Botany, British Museum. With 378 Illustrations. 8vo., 16s.
CROSS AND BEVAN.—CELLULOSE: an Outline of the
Chemistry of the Structural Elements of Plants. With Reference to their
Natural History and Industrial Uses. By Cross and Bevan (C. F. Cross, E.
J. Bevan, and C. Beadle). With 14 Plates. Crown 8vo., 12s. net.
EDMONDS.—Works by HENRY EDMONDS, B.Sc., London.
ELEMENTARY BOTANY, Theoretical and Practical. With
319 Diagrams and Woodcuts. Crown 8vo., 2s. 6d.
BOTANY FOR BEGINNERS. With 85 Illustrations. Fcp.
8vo., 1s. 6d.
KITCHENER.—A YEAR'S BOTANY. Adapted to Home
and School Use. With Illustrations by the Author. By Frances Anna
Kitchener. Crown 8vo., 5s.
[26]
LINDLEY AND MOORE.—THE TREASURY OF BOTANY;
or, Popular Dictionary of the Vegetable Kingdom: with which is incorporated
a Glossary of Botanical Terms. Edited by J. Lindley, M.D., F.R.S., and T.
Moore, F.L.S. With 20 Steel Plates and numerous Woodcuts. Two parts,
fcp. 8vo., 12s.
McNAB.—CLASS-BOOK OF BOTANY. By W. R. McNab.
MORPHOLOGY AND PHYSIOLOGY. With 42 Diagrams.
Fcp. 8vo., 1s. 6d.
CLASSIFICATION OF PLANTS. With 118 Diagrams.
Fcp. 8vo., 1s. 6d.
THOMÉ AND BENNETT.—STRUCTURAL AND PHYSIOLOGICAL
BOTANY. By Dr. Otto Wilhelm Thomé and by Alfred W.
Bennett, M.A., B.Sc., F.L.S. With Coloured Map and 600 Woodcuts.
Fcp. 8vo., 6s.
WATTS.—A SCHOOL FLORA. For the use of Elementary
Botanical Classes. By W. Marshall Watts, D.Sc. Lond. Crown 8vo.,
2s. 6d.
AGRICULTURE AND GARDENING.
ADDYMAN.—AGRICULTURAL ANALYSIS. A Manual of
Quantitative Analysis for Students of Agriculture. By Frank T. Addyman,
B.Sc. (Lond.), F.I.C., Lecturer on Agricultural Chemistry, University College,
Nottingham, etc. With 49 Illustrations. Crown 8vo., 5s. net.
COLEMAN AND ADDYMAN.—PRACTICAL AGRICULTURAL
CHEMISTRY. For Elementary Students, adapted for use in
Agricultural Classes and Colleges. By J. Bernard Coleman, A.R.C.Sc.,
F.I.C., and Frank T. Addyman, B.Sc. (Lond.), F.I.C. With 24 Illustrations.
Crown 8vo., 1s. 6d. net.
LOUDON.—ENCYCLOPÆDIA OF AGRICULTURE; the
Laying-out, Improvement, and Management of Landed Property; the Cultivation
and Economy of the Productions of Agriculture. By J. C. Loudon,
F.L.S. With 1100 Woodcuts. 8vo., 21s.
SORAUER.—A POPULAR TREATISE ON THE PHYSIOLOGY
OF PLANTS. For the use of Gardeners, or for Students of Horticulture
and of Agriculture. By Dr. Paul Sorauer. Translated by F. E.
Weiss, B.Sc., F.L.S. With 33 Illustrations. 8vo., 9s. net.
WEBB.—Works by HENRY J. WEBB, Ph.D., B.Sc. (Lond.),
late Principal of the Agricultural College, Aspatria.
ELEMENTARY AGRICULTURE. A Text-Book specially
adapted to the requirements of the Science and Art Department, the Junior
Examination of the Royal Agricultural Society, and other Elementary Examinations.
With 34 Illustrations. Crown 8vo., 2s. 6d.
AGRICULTURE. A Manual for Advanced Science Students.
With 100 Illustrations. Crown 8vo., 7s. 6d. net.
[27]
WORKS BY JOHN TYNDALL, D.C.L., LL.D., F.R.S.
FRAGMENTS OF SCIENCE: a Series of Detached Essays,
Addresses, and Reviews. 2 vols. Crown 8vo., 16s.
Vol. I.—The Constitution of Nature—Radiation—On Radiant Heat in Relation to the
Colour and Chemical Constitution of Bodies—New Chemical Reactions produced by
Light—On Dust and Disease—Voyage to Algeria to observe the Eclipse—Niagara—The
Parallel Roads of Glen Roy—Alpine Sculpture—Recent Experiments on Fog-Signals—On
the Study of Physics—On Crystalline and Slaty Cleavage—On Paramagnetic
and Diamagnetic Forces—Physical Basis of Solar Chemistry—Elementary
Magnetism—On Force—Contributions to Molecular Physics—Life and Letters of
Faraday—The Copley Medallist of 1870—The Copley Medallist of 1871—Death by
Lightning—Science and the Spirits.
Vol. II.—Reflections on Prayer and Natural Law—Miracles and Special Providences—On
Prayer as a Form of Physical Energy—Vitality—Matter and Force—Scientific Materialism—An
Address to Students—Scientific Use of the Imagination—The Belfast
Address—Apology for the Belfast Address—The Rev. James Martineau and the
Belfast Address—Fermentation, and its Bearings on Surgery and Medicine—Spontaneous
Generation—Science and Man—Professor Virchow and Evolution—The
Electric Light.
NEW FRAGMENTS. Crown 8vo., 10s. 6d.
Contents.—The Sabbath—Goethe's ‘Farbenlehre’—Atoms, Molecules, and Ether Waves—Count
Rumford—Louis Pasteur, his Life and Labours—The Rainbow and its Congeners—Address
delivered at the Birkbeck Institution on October 22, 1884—Thomas Young—Life in the
Alps—About Common Water—Personal Recollections of Thomas Carlyle—On Unveiling the
Statue of Thomas Carlyle—On the Origin, Propagation, and Prevention of Phthisis—Old
Alpine Jottings—A Morning on Alp Lusgen.
LECTURES ON SOUND. With Frontispiece of Fog-Syren, and
203 other Woodcuts and Diagrams in the Text. Crown 8vo., 10s. 6d.
HEAT, A MODE OF MOTION. With 125 Woodcuts and
Diagrams. Crown 8vo., 12s.
LECTURES ON LIGHT DELIVERED IN THE UNITED
STATES IN 1872 AND 1873. With Portrait, Lithographic Plate, and 59
Diagrams. Crown 8vo., 5s.
ESSAYS ON THE FLOATING MATTER OF THE AIR IN
RELATION TO PUTREFACTION AND INFECTION. With 24 Woodcuts.
Crown 8vo., 7s. 6d.
RESEARCHES ON DIAMAGNETISM AND MAGNECRYSTALLIC
ACTION; including the Question of Diamagnetic Polarity. Crown
8vo., 12s.
NOTES OF A COURSE OF NINE LECTURES ON LIGHT,
delivered at the Royal Institution of Great Britain, 1869. Crown 8vo., 1s. 6d.
NOTES OF A COURSE OF SEVEN LECTURES ON
ELECTRICAL PHENOMENA AND THEORIES, delivered at the Royal
Institution of Great Britain, 1870. Crown 8vo., 1s. 6d.
LESSONS IN ELECTRICITY AT THE ROYAL INSTITUTION
1875–1876. With 58 Woodcuts and Diagrams. Crown 8vo., 2s. 6d.
THE GLACIERS OF THE ALPS: being a Narrative of Excursions
and Ascents. An Account of the Origin and Phenomena of Glaciers, and
an Exposition of the Physical Principles to which they are related. With
numerous Illustrations. Crown 8vo., 6s. 6d. net.
FARADAY AS A DISCOVERER. Crown 8vo., 3s. 6d.
[28]
TEXT-BOOKS OF SCIENCE.
PHOTOGRAPHY. By Captain W. de Wiveleslie Abney, C.B., F.R.S., Director
for Science in the Science and Art Department. With 155 Woodcuts. Price
3s. 6d.
THE STRENGTH OF MATERIALS AND STRUCTURES: the Strength of Materials as
depending on their quality and as ascertained by Testing Apparatus; the
Strength of Structures, as depending on their form and arrangement, and
on the materials of which they are composed. By Sir J. Anderson, C.E.,
etc. With 66 Woodcuts. Price 3s. 6d.
RAILWAY APPLIANCES. A Description of Details of Railway Construction
subsequent to the completion of Earthworks and Structures, including a
short Notice of Railway Rolling Stock. By John Wolfe Barry, C.B.,
M.I.C.E. With 218 Woodcuts. Price 4s. 6d.
INTRODUCTION TO THE STUDY OF INORGANIC CHEMISTRY. By William Allen
Miller, M.D., LL.D., F.R.S. With 72 Woodcuts. Price 3s. 6d.
QUANTITATIVE CHEMICAL ANALYSIS. By T. E. Thorpe, F.R.S., Ph.D., Professor
of Chemistry in the Royal College of Science, London. With 88 Woodcuts.
Price 4s. 6d.
QUALITATIVE CHEMICAL ANALYSIS AND LABORATORY PRACTICE. By T. E. Thorpe,
Ph.D., D.Sc., F.R.S., Principal Chemist of the Government Laboratories,
London, and M. M. Pattison Muir, M.A. With Plate of Spectra and 57
Woodcuts. Price 3s. 6d.
INTRODUCTION TO THE STUDY OF CHEMICAL PHILOSOPHY. The Principles of
Theoretical and Systematic Chemistry. By William A. Tilden, D.Sc.,
London, F.R.S., Professor of Chemistry at the Royal College of Science.
With 5 Woodcuts. With or without Answers to Problems. Price 4s. 6d.
ELEMENTS OF ASTRONOMY. By Sir R. S. Ball, LL.D., F.R.S., Lowndean
Professor of Astronomy in the University of Cambridge. With 130 Woodcuts.
Price 6s. 6d.
SYSTEMATIC MINERALOGY. By Hilary Bauerman, F.G.S., Associate of the Royal
School of Mines. With 373 Woodcuts. Price 6s.
DESCRIPTIVE MINERALOGY. By Hilary Bauerman, F.G.S., etc. With 236
Woodcuts. Price 6s.
METALS, THEIR PROPERTIES AND TREATMENT. By C. L. Bloxam and A. K.
Huntington, Professors in King's College, London. With 130 Woodcuts.
Price 5s.
PHYSICAL OPTICS. By R. T. Glazebrook, M.A., F.R.S., Fellow and Lecturer
of Trinity College, and Demonstrator of Physics at the Cavendish
Laboratory, Cambridge. With 183 Woodcuts. Price 6s.
PRACTICAL PHYSICS. By R. T. Glazebrook, M.A., F.R.S., and W. N. Shaw,
M.A. With 134 Woodcuts. Price 7s. 6d.
[29]
PRELIMINARY SURVEY. By Theodore Graham Gribble, Civil Engineer. Including
Elementary Astronomy, Route Surveying, Tacheometry, Curveranging, Graphic
Mensuration, Estimates, Hydrography, and Instruments. With 130
Illustrations, Quantity Diagrams, and a Manual of the Slide-Rule. Price
6s.
ALGEBRA AND_TRIGONOMETRY. By William Nathaniel Griffin, B.D. Price 3s.
6d. Notes on, with Solutions of the more difficult Questions. Price
3s. 6d.
THE STEAM ENGINE. By George C. V. Holmes (Whitworth Scholar), Secretary
of the Institution of Naval Architects. With 212 Woodcuts. Price 6s.
ELECTRICITY AND MAGNETISM. By Fleeming Jenkin, F.R.SS., L. & E., late
Professor of Engineering in the University of Edinburgh. With 177
Woodcuts. Price 3s. 6d.
THE ART OF ELECTRO-METALLURGY, including all known Processes of
Electro-Deposition. By G. Gore, LL.D., F.R.S. With 56 Woodcuts. Price
6s.
TELEGRAPHY. By W. H. Preece, C.B., F.R.S., V.P.Inst., C.E.,
Engineer-in-Chief, and Electrician, Post Office Telegraphs, and Sir J.
Sivewright, M.A., K.C.M.G. With 258 Woodcuts. Price 6s.
THEORY OF HEAT. By J. Clerk Maxwell, M.A., LL.D., Edin., F.R.SS., L.
& E. New Edition. With Corrections and Additions by Lord Rayleigh,
Sec. R. S. With 38 Woodcuts. Price 4s. 6d.
TECHNICAL ARITHMETIC AND MENSURATION. By Charles W. Merrifield, F.R.S.
Price 3s. 6d. Key, by the Rev. John Hunter, M.A. Price 3s. 6d.
THE STUDY OF ROCKS, an Elementary Text-Book of Petrology. By Frank
Rutley, F.G.S., of Her Majesty's Geological Survey. With 6 Plates and 88
Woodcuts. Price 4s. 6d.
WORKSHOP APPLIANCES, including Descriptions of some of the Gauging and
Measuring Instruments—Hand-Cutting Tools, Lathes, Drilling,
Planing, and other Machine Tools used by Engineers. By C. P. B. Shelley,
M.I.C.E. With an additional Chapter on Milling. By R. R. Lister. With 323
Woodcuts. Price 5s.
ELEMENTS OF MACHINE DESIGN. By W. Cawthorne Unwin, F.R.S., B.Sc.,
M.I.C.E. Part I. General Principles, Fastenings, and Transmissive
Machinery. With 304 Woodcuts. Price 6s. Part II. Chiefly on Engine
Details. With 174 Woodcuts. Price 4s. 6d.
STRUCTURAL AND PHYSIOLOGICAL BOTANY. By Dr. Otto Wilhelm Thomé, Rector of
the High School, Cologne, and A. W. Bennett, M.A., B.Sc., F.L.S. With 600
Woodcuts and a coloured Map. Price 6s.
PLANE AND SOLID GEOMETRY. By H. W. Watson, M.A., formerly Fellow of
Trinity College, Cambridge. Price 3s. 6d.
[30]
ADVANCED SCIENCE MANUALS.
∵ Written specially to meet the requirements of the ADVANCED STAGE
of Science Subjects as laid down in the Syllabus of the Directory of the
SCIENCE AND ART DEPARTMENT, SOUTH KENSINGTON.
BUILDING CONSTRUCTION. By the Author of ‘Rivington's Notes on
Building Construction’. With 385 Illustrations and an Appendix of
Examination Questions. Crown 8vo., 4s. 6d.
THEORETICAL MECHANICS. Solids, including Kinematics, Statics, and
Kinetics. By A. Thornton, M.A., F.R.A.S., With 220 Illustrations, 130
Worked Examples, and over 900 Examples from Examination Papers, etc.
Crown 8vo., 4s. 6d.
HEAT. By Mark R. Wright, Hon. Inter. B.Sc. Lond. With 136 Illustrations
and numerous Examples and Examination Papers. Crown 8vo., 4s. 6d.
LIGHT. By W. J. A. Emtage, M.A. With 232 Illustrations. Cr. 8vo., 6s.
MAGNETISM AND ELECTRICITY. By Arthur William Poyser, M.A. With 317
Illustrations. Crown 8vo., 4s. 6d.
INORGANIC CHEMISTRY, THEORETICAL AND PRACTICAL. A Manual for Students in
Advanced Classes of the Science and art Department. By William Jago,
F.C.S., F.I.C. With Plate of Spectra and 78 Woodcuts. Crown 8vo., 4s.
6d.
GEOLOGY: a Manual for Students in Advanced Classes and for General
Readers. By Charles Bird, B.A. (Lond.), F.G.S. With over 300
Illustrations, a Geological Map of the British Isles (coloured), and a
set of Questions for Examination. Crown 8vo., 7s. 6d.
HUMAN PHYSIOLOGY: a Manual for Students in advanced Classes of the
Science and Art Department. By John Thornton, M.A. With 268
Illustrations, some of which are Coloured, and a set of Questions for
Examination. Crown 8vo., 6s.
PHYSIOGRAPHY. By John Thornton, M.A. With 6 Maps, 180 Illustrations, and
Coloured Plate of Spectra. Crown 8vo., 4s. 6d.
AGRICULTURE. By Dr. Henry J. Webb, Ph.D., B.Sc. With 100 Illustrations.
Crown 8vo., 7s. 6d. net.
ELEMENTARY SCIENCE MANUALS.
PRACTICAL, PLANE, AND SOLID GEOMETRY, including Graphic Arithmetic. By
I. H. Morris. Fully Illustrated with Drawings prepared specially for the
book. Crown 8vo., 2s. 6d.
GEOMETRICAL DRAWING FOR ART STUDENTS. Embracing Plane Geometry and its
Applications, the Use of Scales, and the Plans and Elevations of Solids,
as required in Section I. of Science Subject I. By I. H. Morris. Crown
8vo., 1s. 6d.
TEXT-BOOK ON PRACTICAL, SOLID, OR DESCRIPTIVE GEOMETRY. By David Allan
Low (Whitworth Scholar). Part I. Crown 8vo., 2s. Part II. Crown 8vo.,
3s.
AN INTRODUCTION TO MACHINE DRAWING AND DESIGN. By David Allan Low
(Whitworth Scholar). With 97 Illustrations and Diagrams. Crown 8vo.,
2s.
[31]
BUILDING CONSTRUCTION. By Edward J. Burrell, Second Master of the
Technical School of the People's Palace, Mile End. With 308 Illustrations
and Working Drawings. Crown 8vo., 2s. 6d.
AN ELEMENTARY COURSE OF MATHEMATICS. Containing Arithmetic; Euclid (Book
I., with Deductions and Exercises); and Algebra. Crown 8vo., 2s. 6d.
THEORETICAL MECHANICS. Including Hydrostatics and Pneumatics. By J. E.
Taylor, M.A., B.Sc. With numerous Examples and Answers, and 175 Diagrams.
Crown 8vo., 2s. 6d.
THEORETICAL MECHANICS—SOLIDS. By J. E. Taylor, M.A., B.Sc. With 163
Illustrations, 120 Worked Examples, and over 500 Examples from
Examination Papers, etc. Crown 8vo., 2s. 6d.
THEORETICAL MECHANICS—FLUIDS. By J. E. Taylor, M.A., B.Sc. With 122
Illustrations, numerous Worked Examples, and about 500 Examples from
Examination Papers, etc. Crown 8vo., 2s. 6d.
A MANUAL OF MECHANICS: an Elementary Text-Book for Students of Applied
Mechanics. With 138 Illustrations and Diagrams, and 188 Examples taken
from the Science Department Examination Papers, with Answers. By T. M.
Goodeve, M.A. Fcp. 8vo., 2s.
SOUND, LIGHT, AND HEAT. By Mark. R. Wright, Hon. Inter. B.Sc., London.
With Examples, Examination Papers, and 165 Illustrations. Crown 8vo.,
2s. 6d.
PHYSICS. Alternative Course. By Mark R. Wright, Hon. Inter. B.Sc.,
London. With Examples, Examination Papers, and 242 Illustrations. Crown
8vo., 2s. 6d.
ELEMENTARY PRACTICAL CHEMISTRY: a Laboratory Manual for Use in Organised
Science Schools. By G. S. Newth, F.I.C., F.C.S., Demonstrator in the
Royal College of Science, London; Assistant Examiner in Chemistry,
Science and Art Department. With 108 Illustrations and 254 Experiments.
Crown 8vo. Price 2s. 6d.
ELEMENTARY PRACTICAL PHYSICS: a Laboratory Manual for Use in Organised
Science Schools. By W. Watson, B.Sc. Demonstrator in Physics in the Royal
College of Science, London; Assistant Examiner in Physics, Science and
Art Department. With 119 Illustrations and 193 Exercises. Crown 8vo.
Price 2s. 6d.
MAGNETISM AND ELECTRICITY. By A. W. Poyser, M.A. With Examination Papers
and 235 Illustrations. Crown 8vo., 2s. 6d.
PROBLEMS AND SOLUTIONS IN ELEMENTARY ELECTRICITY AND MAGNETISM. Embracing
a Complete Set of Answers to the South Kensington Papers for the Years
1885–1894, and a Series of Original Questions. By W. Slingo and
A. Brooker. With 67 Illustrations. Crown 8vo., 2s.
INORGANIC CHEMISTRY, THEORETICAL AND PRACTICAL. With an Introduction to
the Principles of Chemical Analysis. By William Jago, F.C.S., F.I.C. With
63 Woodcuts and numerous Questions and Exercises. Fcp. 8vo., 2s. 6d.
AN INTRODUCTION TO PRACTICAL INORGANIC CHEMISTRY. By William Jago,
F.C.S., F.I.C. With Illustrations. Crown 8vo., 1s. 6d.
PRACTICAL CHEMISTRY: the Principles of Qualitative Analysis. By William
A. Tilden, D.Sc. With Illustrations. Fcp. 8vo., 1s. 6d.
ELEMENTARY CHEMISTRY, Inorganic and Organic. By William S. Furneaux. With
Examination Questions, and 65 Illustrations. Crown 8vo., 2s. 6d.
ORGANIC CHEMISTRY: the Fatty Compounds. By R. Lloyd Whiteley, F.I.C.,
F.C.S. With 45 Illustrations. Crown 8vo., 3s. 6d.
ELEMENTARY GEOLOGY. By Charles Bird, B.A., F.G.S. With Coloured
Geological Map of the British Islands, and 247 Illustrations. Crown 8vo.,
2s. 6d.
[32]
HUMAN PHYSIOLOGY. By William S. Furneaux. With 218 Illustrations. Crown
8vo., 2s. 6d.
BIOLOGY. By John Bidgood, B.Sc. With 226 Illustrations. Crown 8vo., 4s.
6d.
ELEMENTARY BOTANY, THEORETICAL AND PRACTICAL. By Henry Edmonds, B.Sc.,
London. With 319 Woodcuts. Crown 8vo., 2s. 6d.
METALLURGY. By E. L. Rhead, Lecturer on Metallurgy at the Municipal
Technical School, Manchester. With 94 Illustrations. Crown 8vo., 3s.
6d.
STEAM. By William Ripper, Member of the Institution of Mechanical
Engineers, Professor of Mechanical Engineering in the Sheffield Technical
School. With 142 Illustrations. Crown 8vo., 2s. 6d.
ELEMENTARY PHYSIOGRAPHY. By John Thornton, M.A. With 12 Maps and 247
Illustrations. Crown 8vo., 2s. 6d.
AGRICULTURE. By Henry J. Webb, Ph.D., B.Sc. (Lond.). Late Principal of
the Agricultural College, Aspatria. With 34 Illustrations. Crown 8vo.,
2s. 6d.
HYGIENE. By J. L. Notter, M.A., M.D., Fellow and Member of Council of the
Sanitary Institute of Great Britain, Examiner in Hygiene, Science and Art
Department; Examiner in Public Health in the University of Cambridge and
in the Victoria University, Manchester; and R. H. Firth, F.R.C.S.,
Assistant Professor of Hygiene, in the Army Medical School, Netley. With
95 Illustrations. Crown 8vo., 3s. 6d.
THE LONDON SCIENCE CLASS-BOOKS.
Edited by G. Carey Foster, F.R.S., and by Sir Philip Magnus, B.Sc., B.A.,
of the City and Guilds of London Institute.
ASTRONOMY. By Sir Robert Stawell Ball, LL.D., F.R.S. 41 Diagrams. 1s.
6d.
MECHANICS. By Sir Robert Stawell Ball, LL.D., F.R.S. 89 Diagrams. 1s.
6d.
THE LAWS OF HEALTH. By W. H. Corfield, M.A., M.D., F.R.C.P. With 22
Illustrations. 1s. 6d.
MOLECULAR PHYSICS AND SOUND. By Fred. Guthrie, F.R.S. 91 Diagrams. 1s.
6d.
GEOMETRY, CONGRUENT FIGURES. By O. Henrici, Ph.D., F.R.S. With 141
Diagrams. 1s. 6d.
ZOOLOGY OF THE INVERTEBRATE ANIMALS. By Alexander Macalister, M.D. With
59 Diagrams. 1s. 6d.
ZOOLOGY OF THE VERTEBRATE ANIMALS. By Alexander Macalister, M.D. With 77
Diagrams. 1s. 6d.
HYDROSTATICS AND PNEUMATICS. By Sir Philip Magnus, B.Sc., B.A. 79
Diagrams. 1s. 6d. (To be had also with Answers, 2s.). The Worked
Solutions of the Problems. 2s.
BOTANY. Outlines of the Classification of Plants. By W. R. McNab, M.D.
With 118 Diagrams. 1s. 6d.
BOTANY. Outlines of Morphology and Physiology. By W. R. McNab, M.D. With
42 Diagrams. 1s. 6d.
THERMODYNAMICS. By Richard Wormell, M.A., D.Sc. 41 Diagrams. 1s. 6d.
Transcriber's Notes
The half-title was deleted.
Spelling and punctuation of the original text were taken over
and obvious errors have been corrected silently.
Inconsistant hyphenation was kept as it is.
This book contains links to another book in the Project Gutenberg collection.
Although we verify the correctness of these links at the time of posting,
these links may not work, for various reasons, for various people, at various times.
*** END OF THE PROJECT GUTENBERG EBOOK 54210 ***